Figure 2. Regulation of signaling receptors.
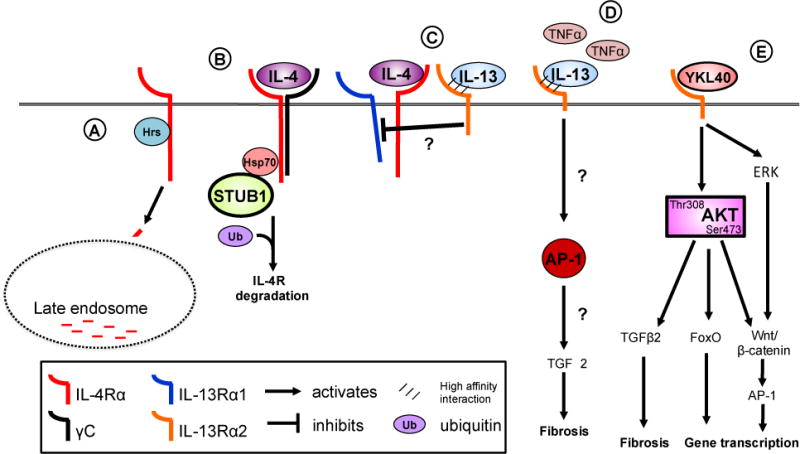
IL-4Rα expression is regulated in the steady state by Hepatocyte growth factor-regulated tyrosine substrate (Hrs) (A). Hrs binding targets the IL-4Rα to the late endosome for degradation. Downregulation of IL-4Rα occurs through STUB1 binding the cytoplasmic domain of the receptor through Hsp70 (B). Subsequent ubiquitination and degradation the IL-4Rα reduces cell surface expression and cellular responsiveness to IL-4. The IL-13Rα2 acts as a negative regulator of IL-4- but not IL-13-induced signaling through the type II IL-4 receptor (C). The IL-13Rα2 acts as a negative regulator of IL-4- but not IL-13-induced signaling through the type II IL-4 receptor although the precise mechanisms of this inhibition are not understood. IL-13Rα2 expression is induced by TNFα in fibroblasts and leads to AP-1 driven pro-fibrotic reprogramming (D). YKL40 binds binds to the IL-13Rα2 chain leading to serine 473 phosphorylation in addition to triggering ERK signaling (E). AKT phosphorylation following binding of YKL40 to IL-13Rα2 activates a myriad of signaling pathways including FoxO1/3 and the Wnt/b-catenin pathway. Hepatocyte growth factor-regulated tyrosine substrate, Hrs: STIP1 homology and U-Box containing protein 1, STUB1; heat shock protein 70, Hsp70; forkhead box protein O, FoxO; transforming growth factor-β, TGF-β; activator protein-1, AP-1.
