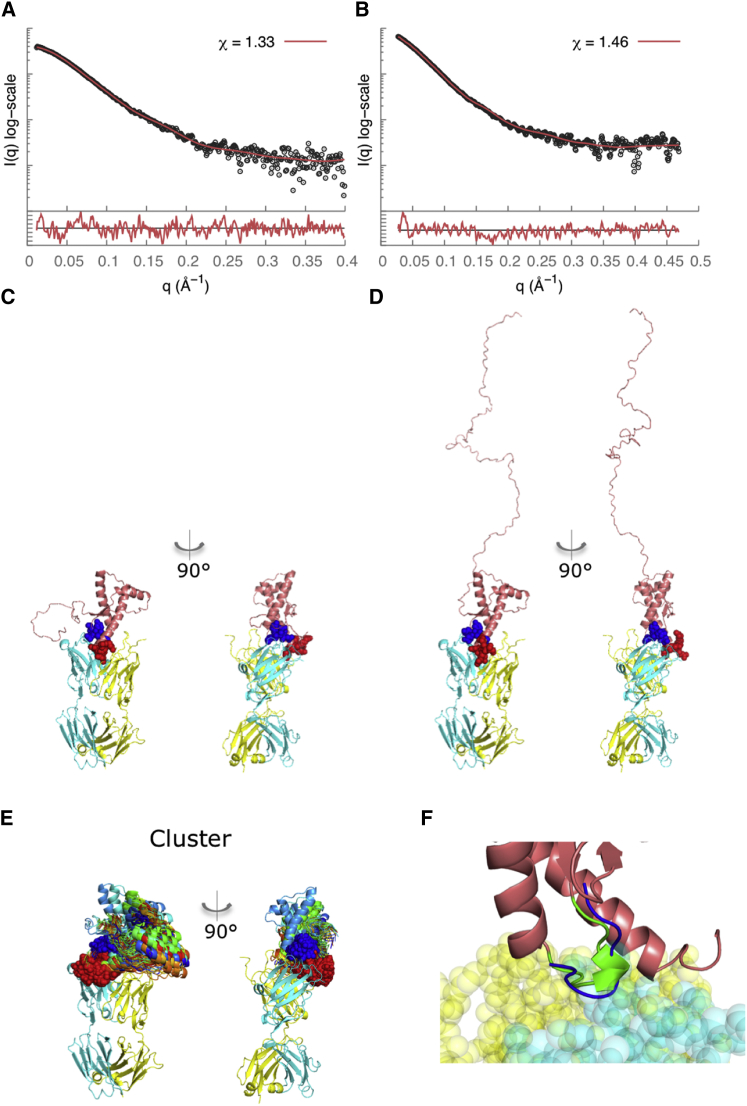
Figure 6.
SAXS data analysis and modeling for the recPrP(89–230)-Fab-R1 (left column) and recPrP(23–230)-Fab-R1 (right column) complexes. The SAXS profile fits (A and B) and the corresponding best-scoring structural models (C and D) are shown for each of the samples. The lower plots in (A) and (B) show the residuals defined as (Iexp(q) − Icalc(q))/σexp(q), corresponding to the difference between the experimental and computed intensities weighted by the experimental uncertainty. (E) The cluster of the top-scoring models is shown. The heavy chain of the Fab is shown in cyan, the light chain in yellow, and the epitope residues in red spacefill; the rigid loop is shown in blue. (F) Our structural model reveals interactions of the α2-β2 loop of PrPC (residues 165–175) with Fab-R1 (spacefill model) (41–43). This loop region in murine PrPC exists in two structural states, a highly populated state that forms a 310-helical turn (green) and a minor state that forms a type-I β-turn (blue) (41,43). To see this figure in color, go online.