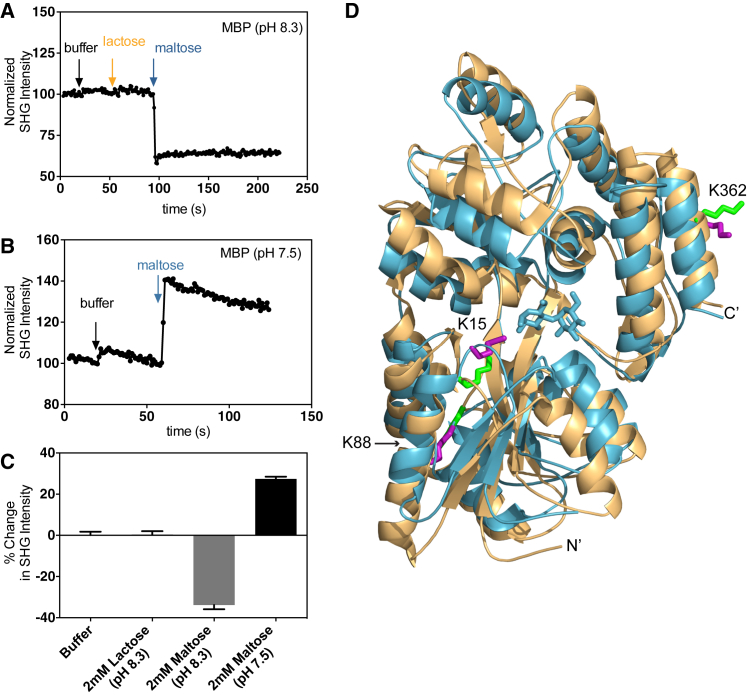
Figure 3.
MBP. (A) Representative time course of an SHG signal during compound addition to MBP labeled at pH 8.3. The arrows denote the time of injection of buffer, lactose, and maltose. (B) Representative time course of an SHG signal during compound addition to MBP labeled at pH 7.5. The arrows denote the time of injection of buffer and maltose. (C) Summary of the percent change in SHG signal observed after addition of buffer, lactose, and maltose to MBP at a 2-min endpoint (N ≥ 3). (D) Overlay of the crystal structures of MBP with maltose (blue; PDB ID: 1ANF) and without maltose (tan; PDB ID: 1JW4) bound. Maltose is shown in blue. Modified lysine residues identified by MS are shown as sticks in green on the unbound structure and in purple on the bound structure. The labeled lysines are in different conformations in the two crystal structures. For this comparison, the structures were aligned at their N-terminal domains. The His-tag and thus the site of immobilization of the protein are at the N-terminus.