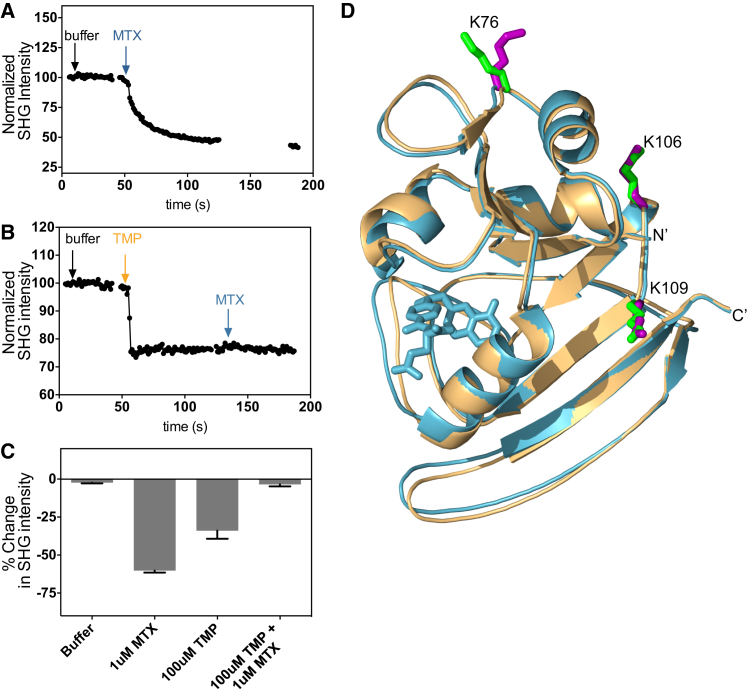
Figure 4.
Amine-labeled DHFR. (A) Representative kinetic trace for MTX addition to lysine-labeled DHFR. Arrows denote the time of addition of buffer and MTX. (B) Representative kinetic trace for a TMP-MTX competition experiment with lysine-labeled DHFR. Arrows denote the time of addition of buffer, TMP, and MTX. (C) Summary of percent change of SHG signal observed after addition of buffer, MTX, and TMP to lysine-labeled DHFR at a 2-min endpoint (N ≥ 4). (D) Crystal structures of DHFR holoenzyme with (blue; PDB ID: 1RB3) and without (tan; PDB ID: 1RX1) MTX bound. Labeled residues identified by MS are shown as sticks in green for DHFR without MTX, and in purple for DHFR bound to MTX. MTX is represented in blue. NADPH (100 μM) was present in all experiments.