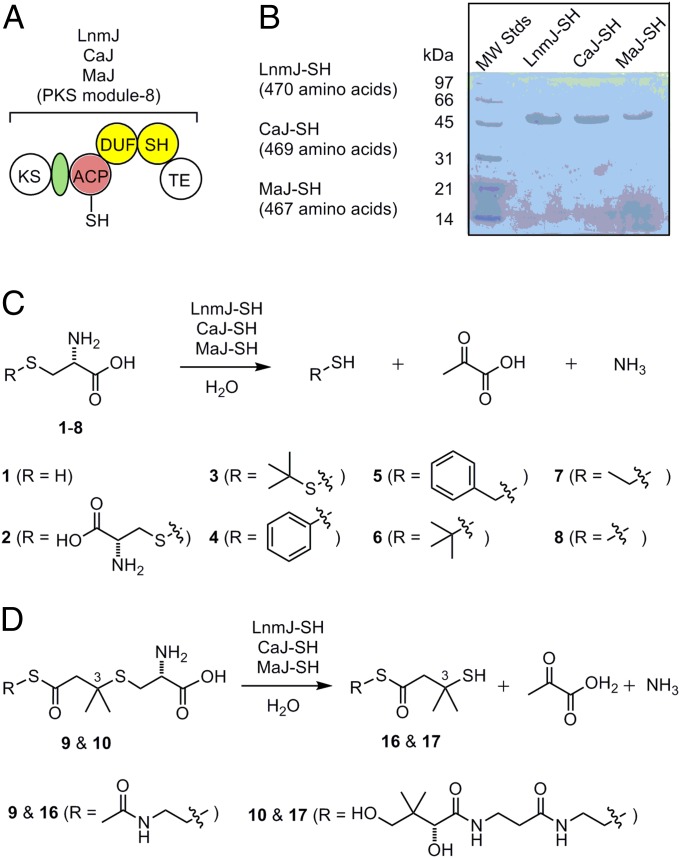
Fig. 3.
LnmJ-SH, CaJ-SH, and MaJ-SH as a new family of PKS domains that catalyzes C-S bond cleavage. (A) Domain organization and architectural conservation of the PKS modules featuring the DUF and SH domains reported in this study. (B) SDS/PAGE (12%) of the purified SH domains (calculated molecular mass, including the extra amino acids from expression vector, in daltons): LnmJ-SH (49,609.6), CaJ-SH (49,699.9), and MaJ-SH (48,539.8). (C) LnmJ-SH, CaJ-SH, and MaJ-SH catalyze C-S bond cleavage using l-cysteine (1) and l-cysteine S-modified analogs (2–8) as substrates. (D) LnmJ-SH, CaJ-SH, and MaJ-SH catalyze C-S bond cleavage using N-acetylcysteamine and pantetheine thioester of the l-cysteine-polyketide adducts 9 and 10 as substrate mimics of the ACP-tethered growing polyketide intermediate in LNM biosynthesis, affording the corresponding products 16 and 17 featuring a thiol moiety at C-3 (Fig. 1).