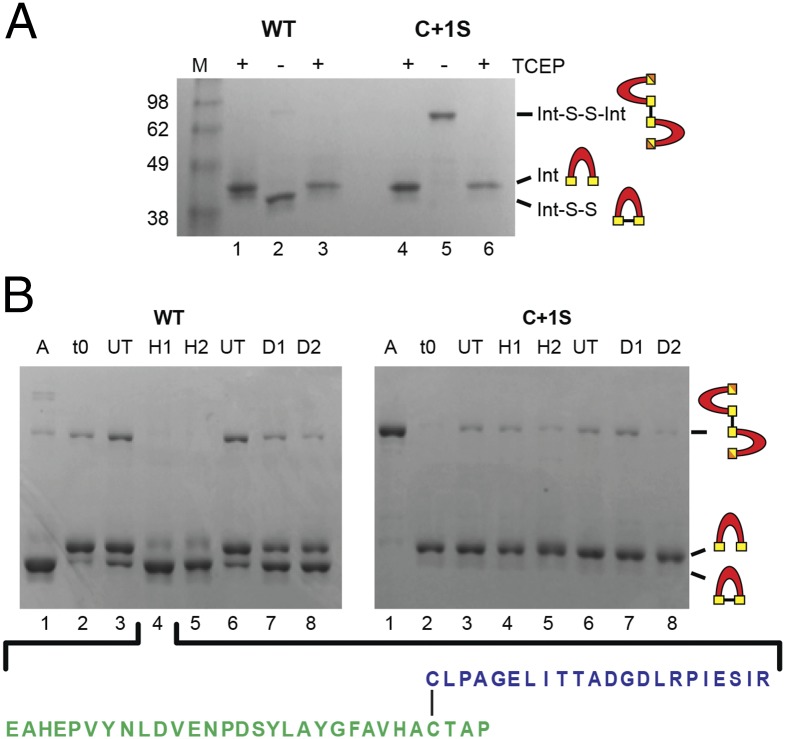
Fig. 4.
Oxidative modifications of the SufB intein. (A) Intra- and intermolecular disulfide bond formation. Purified WT and C+1S SufBi-PC were run with TCEP (+, lanes 1 and 4). Upon TCEP removal, WT forms intramolecular (Int-S-S) and intermolecular (Int-S-S-Int) disulfide bonds (–, lane 2), as visualized by band shifts. The C+1S precursor forms only the intermolecular disulfide bond (–, lane 5). TCEP treatment reversed disulfide bond formation to yield reduced intein (+, lane 3 and 6). LC-MS confirmation presented in Fig. S4A. (B) ROS and RNS promote disulfide bond formation only in WT precursor. A, Aerobic; t0, protein capped with iodoacetamide immediately after the removal of TCEP; UT, untreated; H1, 10×-fold [H2O2] over protein; H2, 100×-fold [H2O2]; D1, 10×-fold [DEA]; D2, 100×-fold [DEA]. The C1−C+1 intramolecular disulfide-bonded peptide (lane 4) was further validated by LC-MS/MS (Fig. S4B). The peptide coverage is shown below and in Fig. S4B.