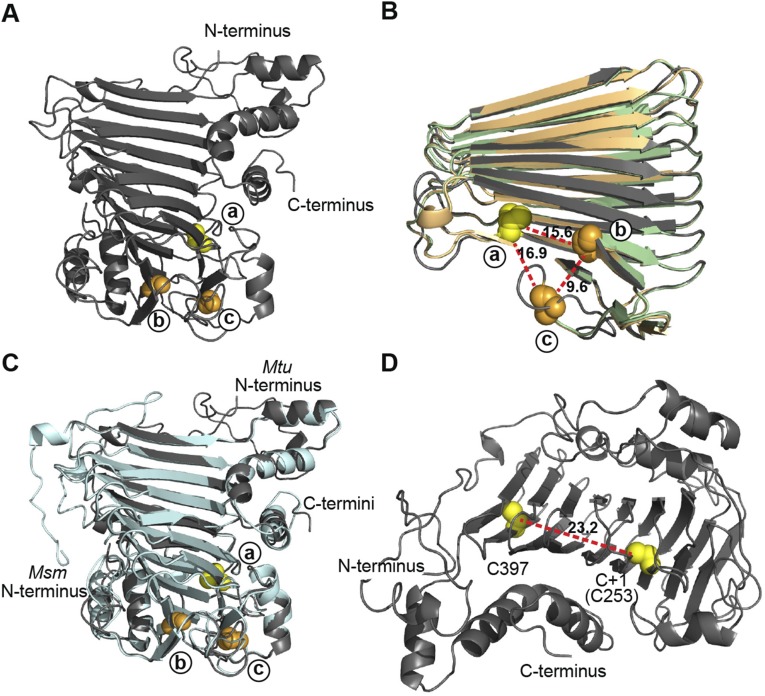
Fig. S2.
Structure modeling of SufB exteins. (A) Full structure model of M. tuberculosis SufB exteins. N- and C-termini are shown. (B) Overlay of SufB exteins representing the three distinct insertion sites. The SufB extein models of M. tuberculosis (dark gray; site a), M. leprae (light green; site b), and M. xenopi (light orange; site c) were aligned and simplified to the core β-helix. Alignment shows a high level of structural conservation and clustering of the insertion sites in 3D space. The distance between +1 residues was measured, based on the M. tuberculosis extein model: site a to site b 15.6 Å; site b to site c 9.6 Å; site c to site a 16.9 Å. (C) Overlay of full SufB extein models from intein-containing M. tuberculosis (dark gray) and inteinless M. smegmatis (light cyan). Alignment shows a high level of structural conservation and the conservation of the +1 residues at the three insertion sites. (D) Distance between cysteines in the SufB exteins. The distance between the two exclusive cysteines (C397 and C+1[C253]) in the M. tuberculosis SufB exteins was measured to be 23.2 Å. This distance suggests that C397 and C+1 are not cooperatively involved in [Fe-S] cluster coordination. All displayed models were made using Phyre2 servers. PyMOL was used to manipulate models and generate distances. Cysteines are indicated as yellow spheres and, with the exception of C397 in D, represent the C+1 of the site a insertions. Serines are shown as orange spheres and represent the S+1 of either the site b or site c insertions as indicated.