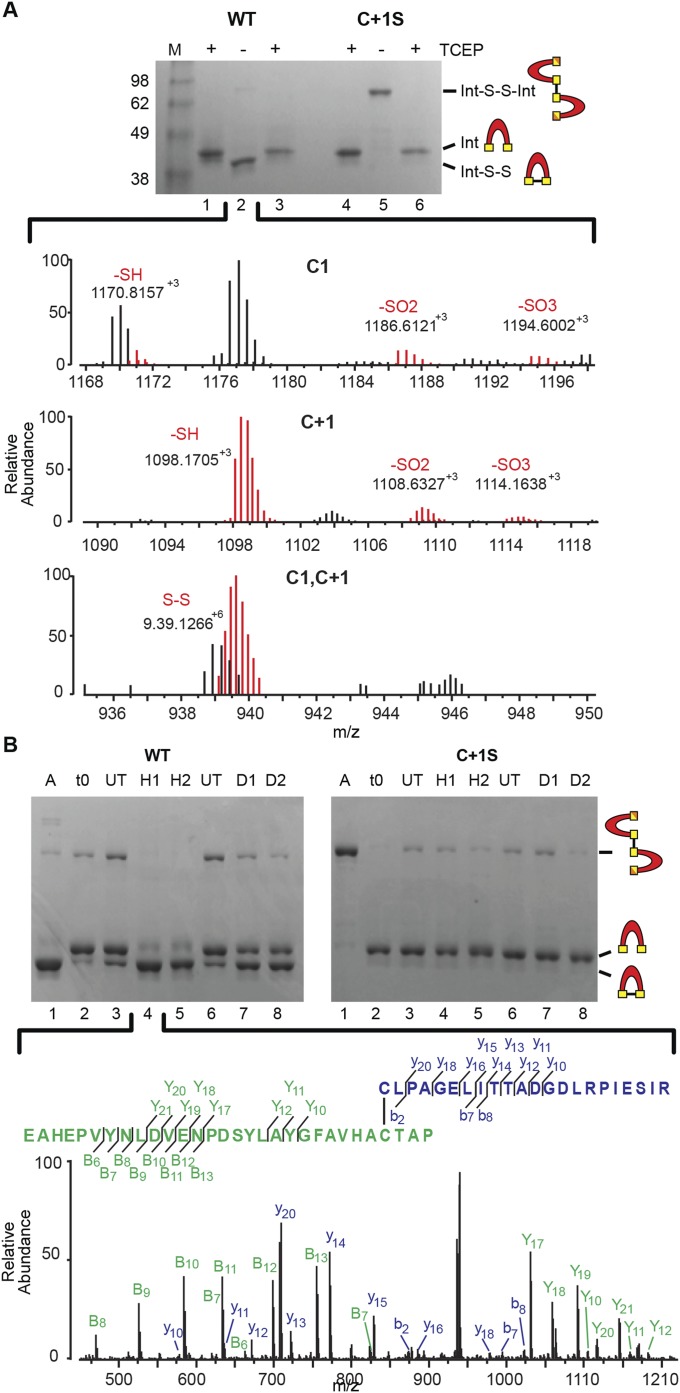
Fig. S4.
Oxidative modifications of the SufB intein confirmed by mass spectrometry. (A) Intra- and intermolecular disulfide bond formation. WT precursor without TCEP (lane 2) was trypsin-digested and analyzed by LC-MS. The Top and Middle show the various oxidative states of C1 and C+1, respectively. The Bottom shows the disulfide bonded C1-C+1 peptide, which is colored red to distinguish from other overlapping peaks. Gels are described in Fig. 4A. (B) ROS and RNS promote disulfide bond formation only in WT precursor. The C1−C+1 intramolecular disulfide-bonded peptide identified in H2O2 -treated WT SufBi-PC (H1) was further validated by LC-MS/MS (lane 4). The peptide coverage is shown in the Inset. The spectra represent the peaks generated by fragmentation of the C1−C+1 disulfide-bonded peptide. The “y” and “b” ions correspond to C1 peptide fragments, and the “Y” and “B” ions correspond to C+1 peptide fragments. Gels are described in Fig. 4B. Disulfide bond formation between C1 and C+1 was detected with both treatments, although other NO-based modifications were not detected, likely due to the labile nature of these modifications (41–43).