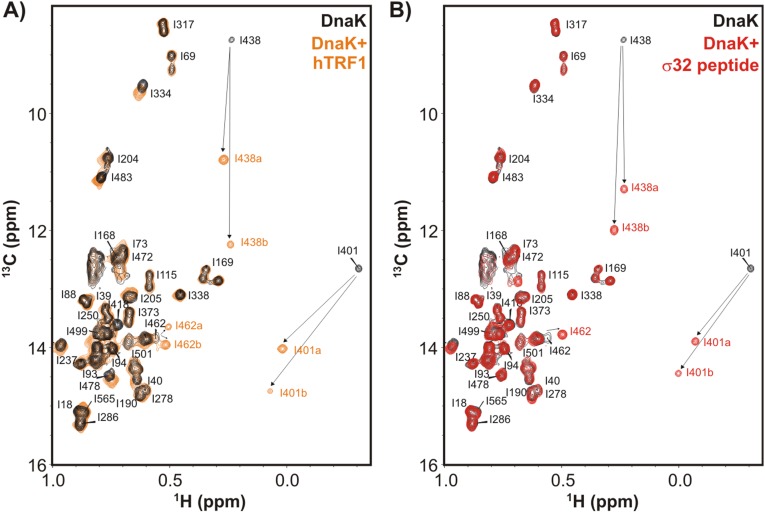
Fig. S2.
hTRF1 binds DnaK similarly to the well-studied canonical substrate σ32 peptide. (A and B) Ile δ1 region of a 1H-13C HMQC spectrum of ILVM-13CH3/2H ADP-DnaK in the absence (black) and presence (orange) of 2H/12C hTRF1 (A) or without (black) and with (red)1H/12C σ32 peptide (B), 35 °C. The residues of DnaK near the substrate binding site move as indicated by arrows upon addition of substrate. Duplicate peaks are observed for a number of methyl groups, as denoted by a and b in the figure. Assignments of ADP-DnaK are those from a previously published report (18).