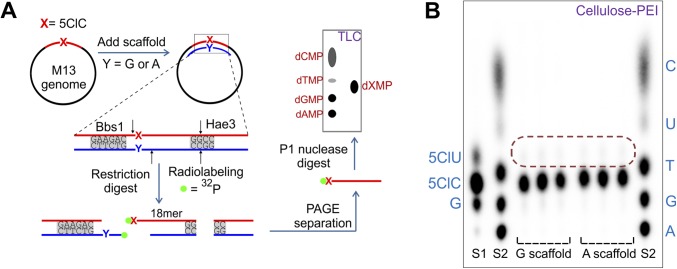
Fig. S4.
Purity analysis of the 5ClC-containing genome. (A) Lesion integrity assay. Scaffolds containing G or A opposite the lesion site and restriction enzymes (with the restriction sites indicated) were used to isolate a 32P-radiolabeled monophosphate of the base X (base at the lesion site, i.e., 5ClC) and evaluate its identity and purity on TLC (Cellulose-PEI). (B) The result of the lesion integrity assay applied to an M13 genome containing 5ClC. Two mixtures of deoxynucleotide monophosphate standards, S1 (5ClU, 5ClC, G) and S2 (C, U, T, G, A) were loaded on the TLC plate alongside three independent replicates for each scaffold. The area encircled by the red dotted line indicates the location where any putative 5ClU contaminant would appear. Quantification revealed that the constructed genomes contained >99% 5ClC at the lesion site.