Fig. 1.
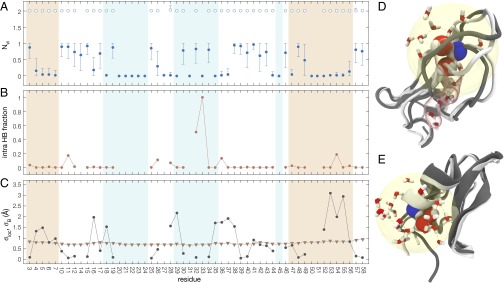
Structure of the O state for the amides in BPTI. (A) Primary water–oxygen coordination number, , of amide hydrogen for amides in O (open symbols) and C (solid symbols) states. (B) Probability that an amide hydrogen with has a polar protein atom from a nonneighbor residue within 2.6 Å. (C) O/C rmsd for atoms within 7 Å of amide N (circles) and crystallographic root-mean-square fluctuation for same set of atoms (triangles). In A–C, the background shading indicates helix (beige) and β-sheet (light blue) structure. Snapshots of O states for the amides of Gly36 (D) and Arg53 (E), with the N–H group and the two primary waters in space-filling and other waters within a 7-Å sphere (yellow) in stick representation. The backbone conformation is shown for the selected O-state frame (dark gray) and for the first C-state frame in the trajectory (light gray). Also shown for Gly36 is a five-water chain in a tunnel (translucent red) connecting one of the primary waters with the surface.
