Abstract
Bone sialoprotein (BSP) is an acidic phosphoprotein with collagen-binding, cell attachment, and hydroxyapatite-nucleating properties. BSP expression in mineralized tissues is upregulated at onset of mineralization. Bsp-null (Bsp-/-) mice exhibit reductions in bone mineral density, bone turnover, osteoclast activation, and impaired bone healing. Furthermore, Bsp-/- mice have marked periodontal tissue breakdown, with a lack of acellular cementum leading to periodontal ligament detachment, extensive alveolar bone and tooth root resorption, and incisor malocclusion. We hypothesized that altered mechanical stress from mastication contributes to periodontal destruction observed in Bsp-/- mice. This hypothesis was tested by comparing Bsp-/- and wild-type mice fed with standard hard pellet diet or soft powder diet. Dentoalveolar tissues were analyzed using histology and micro–computed tomography. By 8 wk of age, Bsp-/- mice exhibited molar and incisor malocclusion regardless of diet. Bsp-/- mice with hard pellet diet exhibited high incidence (30%) of severe incisor malocclusion, 10% lower body weight, 3% reduced femur length, and 30% elevated serum alkaline phosphatase activity compared to wild type. Soft powder diet reduced severe incisor malocclusion incidence to 3% in Bsp-/- mice, supporting the hypothesis that occlusal loading contributed to the malocclusion phenotype. Furthermore, Bsp-/- mice in the soft powder diet group featured normal body weight, long bone length, and serum alkaline phosphatase activity, suggesting that tooth dysfunction and malnutrition contribute to growth and skeletal defects reported in Bsp-/- mice. Bsp-/- incisors also erupt at a slower rate, which likely leads to the observed thickened dentin and enhanced mineralization of dentin and enamel toward the apical end. We propose that the decrease in eruption rate is due to a lack of acellular cementum and associated defective periodontal attachment. These data demonstrate the importance of BSP in maintaining proper periodontal function and alveolar bone remodeling and point to dental dysfunction as causative factor of skeletal defects observed in Bsp-/- mice.
Keywords: bone sialoprotein, cementum, periodontal ligament, bone, mechanotransduction, malocclusion
Introduction
Bone sialoprotein (BSP) is a multifunctional phosphoprotein belonging to the SIBLING family (small integrin-binding ligand N-linked glycoprotein; Fisher and Fedarko 2003). BSP localizes primarily to mineralized tissues, including bone, calcified cartilage, dentin, and cementum, where its expression is upregulated at the onset of mineralization (Macneil et al. 1994; McKee et al. 1996). In vitro studies have demonstrated that the integrin-binding RGD sequence in BSP promotes cell attachment, signaling, and migration (Ganss et al. 1999; Goldberg and Hunter 2012). In addition, BSP binds collagen (Tye et al. 2005; Baht et al. 2008) and hydroxyapatite with high affinity (Goldberg et al. 2001) and promotes mineral formation (Hunter and Goldberg 1993). Analysis of the Bsp-/- mouse, during development and repair, revealed decreased growth and mineralization of long bones (Malaval et al. 2008; Malaval et al. 2009; Holm et al. 2015). Lack of BSP also affected osteoclast formation and recruitment negatively, leading to reduced long bone turnover (Malaval et al. 2008; Boudiffa et al. 2010).
Previously, we reported that loss of BSP causes inhibition of functional acellular cementum on molar and incisor tooth roots, hypomineralization of cellular cementum, and detachment and disorganization of the periodontal ligament (PDL; Foster et al. 2013). In contrast to reduced bone turnover reported in femurs and tibias (Malaval et al. 2008), we reported increased osteoclastic resorption in alveolar bone of Bsp-/- mice, associated with increased presence of receptor activator of nuclear factor kappa-B ligand (RANKL) in the periodontium, increased numbers of tartrate resistant acid phosphatase (TRAP)-positive osteoclast-like cells, cervical resorption of molars, substantial alveolar bone loss, and increased incidence of incisor malocclusion (Foster et al. 2013). Collectively, these data support the interpretation that a lack of acellular cementum is the primary defect, leading to PDL detachment, epithelial downgrowth, and alveolar bone destruction. We hypothesized that defective periodontal attachment due to a lack of acellular cementum played a central role in the periodontal breakdown observed in Bsp-/- mice and that forces generated by occlusion contributed to these pathologic changes in dentoalveolar tissues. In this study, we aimed to determine whether reducing masticatory forces through use of soft diet (SD) would ameliorate periodontal dysfunction and malocclusion in Bsp-/- mice. To elucidate the pathologic mechanisms involved, changes in molar and incisor teeth were analyzed by micro–computed tomography (micro-CT) and histology over time.
Materials and Methods
Animals
Animal care followed guidelines of the Canadian Council on Animal Care and Veterinary Services at the University of Western Ontario. Preparation and genotyping of Bsp-/- and wild-type (WT) mice, maintained on a mixed 129/CD1 background, were performed as described previously (Malaval et al. 2008). At 3 wk of age, Bsp-/- and WT littermates, offspring from heterozygous mice mating, were randomly assigned to groups fed a diet (2018 Tekland Global 18% protein; Harlan Laboratories, Indianapolis, IN, USA) either in the form of hard pellets (hard diet [HD]) or soft powder (soft diet [SD]) with access to water ad libitum. Mice were weighed weekly (3 to 9 wk). Experimental end points for histologic and radiographic analysis were 4, 8, and 20 wk.
Blood Chemistry
Animals were anesthetized with isoflurane gas and whole blood collected by cardiac puncture. Blood samples were transferred to BD Vacutainer SST tubes and allowed to coagulate at room temperature for 20 min. After centrifugation at 3,000 g for 10 min at 4°C, serum supernatants were collected and stored at −20°C. Serum biochemistry was analyzed at the National Institutes of Health, Veterinary Services Clinical Chemistry (Bethesda, MD, USA).
Histology
Long bones and mandibles used for micro-CT were harvested, fixed in 10% neutral-buffered formalin solution overnight, and stored in calcium-free Hank’s buffered saline solution. Procedures for histology and analysis of hemimandibles were previously described (Foster 2012).
Micro–computed tomography
Micro-CT analysis of hemimandibles was performed using eXplore Locus SP (GE Medical Systems, London, Ontario, Canada) at 80 kVP, 80 mA, with a 0.508-mm Al filter and exposure time of 1,600 ms/frame at 4 frames/view to obtain 900 views. These images were reconstructed at 13-μm spatial resolution with calibration and data analysis as described previously (Foster et al. 2013). Long bone images were acquired with eXplore Locus Ultra (GE Medical Systems) to obtain 1,000 views at 120 kVP, 20 mA, and 16 ms/view exposure time. Data were reconstructed at 154-μm spatial resolution. Long bone lengths were measured between the tips of the medial condyle and greater trochanter (femurs) or medial malleolus (tibias).
For analysis of hemimandibles, regions of interest (ROIs) were identified at 3 anatomic positions along the incisor: alveolar crest, first molar (mesial root), and third molar. Coronal sections at these positions were used to measure the mineral density, thickness, and areas of incisor enamel, labial dentin, and lingual dentin. For the first molar analysis, ROIs were determined at 3 positions: crown enamel and dentin, as well as root dentin. For all mineral density measurements, 3-dimensional cubical volumes of interest (10 isotropic voxel size) were defined within the ROIs. Three separate measurements were obtained and averaged for each ROI. The 2-dimensional area of interest was defined by manually drawn contours along the boundaries of the incisors.
For mandibular molars, mesial root exposure was determined by comparing the distance between the buccal/lingual alveolar ridge and the cementoenamel junction with the total root length. All measurements were performed at the midline of mesial root of first and second molars. As an indicator of bone loss, the PDL width in the furcation region of first and second molars was measured.
Statistical Analyses
Quantitative data are expressed as mean ± standard error. Statistical analyses between experimental groups were performed with 2-way analysis of variance and Bonferroni posttests with Prism 5 (version 5.03; GraphPad, La Jolla, CA, USA).
Results
SD Normalized Body Weight, Long Bone Length, and Incisor Malocclusion of Bsp-/- Mice
To analyze the role of masticatory forces on the periodontal phenotype of Bsp-/- mice, WT and Bsp-/- mice were assigned to HD and SD groups. From 3 to 9 wk of age, Bsp-/-(HD) mice weighed significantly less than WT(HD) (Fig. 1A), consistent with studies on 8-wk-old (Bouleftour et al. 2014) and 4-mo-old mice (Malaval et al. 2008). However, WT(SD) and Bsp-/-(SD) groups were not statistically different in weight. While weight of WT mice were not significantly different between diets, weight of Bsp-/-(SD) was significantly greater than Bsp-/-(HD) at 3 to 5 wk but not in older mice, suggesting that differences become less pronounced with age. These data suggest that SD, at least partially, rescues the reduced body weight phenotype of Bsp-/- mice. Bsp-/- mice did not exhibit any abnormalities in behavior, fertility, breeding, and nursing capabilities.
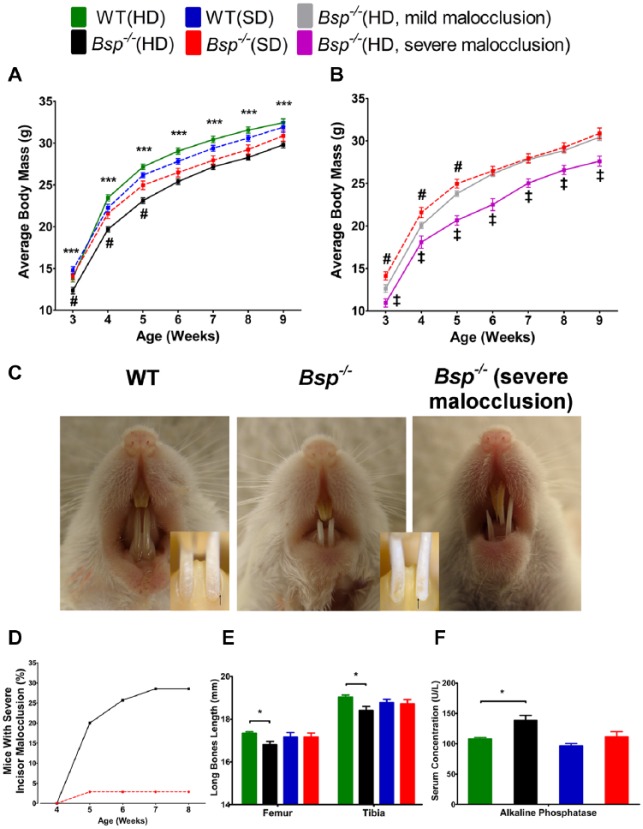
Figure 1.
Soft diet normalizes body weight, bone length, and incisor malocclusion in Bsp-/-mice. (A) Between 3 and 9 wk, Bsp-/- mice fed with hard diet (HD) weighed significantly less (***P < 0.001) than did the wild-type (WT) counterpart, WT(HD) (n = 35 to 58 for weeks 3 to 8; n = 27 at week 9). However, WT and Bsp-/- mice fed soft diet (SD) were not statistically different in weight. Between 3 and 5 wk, Bsp-/-(SD) weighed significantly greater (#; P < 0.05) than Bsp-/-(HD) but not among older mice. Note: The average body mass for Bsp-/-(HD) mice includes all mice regardless of degree of malocclusion. (B) Bsp-/-(HD) with severe malocclusion weighed significantly less (‡; P < 0.05) than Bsp-/-(HD) with milder malocclusion, suggesting that this phenotype might be caused by dental dysfunction and malnutrition. (C) Bsp-/- mice, regardless of diet, developed some degree of malocclusion associated with labial rotation of the incisors, observed by altered location of the cementoenamel junction (black arrows in insets). Severe malocclusion of maxillary and mandibular incisors is prominent in Bsp-/-(HD). (D) Over 8 wk, 10 of 35 (~30%) Bsp-/-(HD) mice developed severe incisor malocclusion, a feature evident in only 1 of 35 (~3%) Bsp-/-(SD) mice. (E) In the HD groups, femurs and tibias were significantly shorter in 8-wk-old Bsp-/- mice vs. WT mice; however, no differences were apparent between the 2 SD groups (n = 6). (F) The increased serum alkaline phosphatase activity in Bsp-/- vs. WT was normalized in the SD groups (n = 5). For panels A, B, E, and F, values shown represent means ± SEM. *P < 0.05.
Bsp-/- mandibular incisors featured chalky white enamel, in contrast to the normal yellow-brown coloration in WT and heterozygous mice (Fig. 1C). Regardless of diet, Bsp-/- mice developed some degree of malocclusion, with mandibular incisors rotated in a lingual-to-labial direction when compared with WT (Fig. 1C insets). Over the course of 8 wk, ~30% of Bsp-/-(HD) but only ~3% of Bsp-/-(SD) developed severe malocclusion, affecting maxillary and mandibular incisors, causing overgrowth, and resulting in dysfunction (Fig. 1C, D). WT and heterozygous mice fed HD did not develop malocclusion (data not shown). Bsp-/-(HD) mice with severe incisor malocclusion weighed significantly less than Bsp-/-(HD) with milder incisor malocclusion (Fig. 1B), suggesting that severe malocclusion, with corresponding malnutrition, may contribute to the observed bone phenotype.
Previous studies reported shortening of long bones in Bsp-/- mice fed HD at 4 and 16 mo of age (Malaval et al. 2008), consistent with our findings at 8 wk (Fig. 1E). However, with the SD, femur and tibia lengths of Bsp-/- and WT mice were not significantly different. Furthermore, increased serum alkaline phosphatase (ALP) activity, a marker of skeletal activity in Bsp-/- mice (Foster et al. 2013), was also normalized with SD (Fig. 1F). Other blood chemistry markers—including calcium, phosphorus, magnesium, sodium, chloride, glucose, albumin, and protein triglycerides—were similar for all groups (data not shown). Thus, SD normalized body weight, long bone length, and serum ALP and eliminated severe malocclusion in Bsp-/- mice.
Altered Dentin and Enamel Formation in Incisors of Bsp-/- Mice
At 4 wk of age, Bsp-/- incisors appeared similar to WT (Fig. 2A). However, by 8 wk, micro-CT and histology revealed increased dentin thickness and decreased pulp volume in Bsp-/- mouse incisors as compared with WT (Figs. 2B, C, E, G, I, and 3A, B). Incisor dentin thickness measured at the first and third molars increased by 85% in Bsp-/- mice (Fig. 3B), whereas enamel thickness was normal at all positions (data not shown). Furthermore, the incisors featured enhanced mineralization of dentin and enamel in the unerupted portion vs. WT incisors (Figs. 2B, 3C, D). At the site of eruption (at the alveolar crest), dentin and enamel mineral densities were equivalent in Bsp-/- and WT. Mineral density of Bsp-/- molar crown and root dentin were comparable with that of WT; however, enamel mineral density was elevated by 12.4% (Fig. 3E).
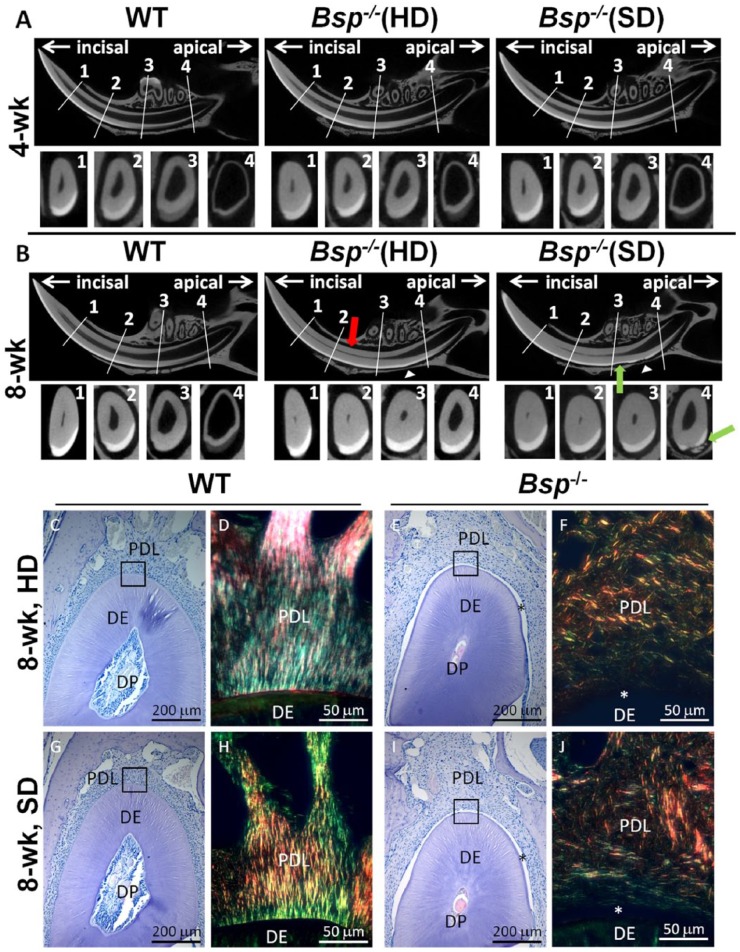
Figure 2.
Altered incisor dentin and enamel formation in Bsp-/- mice. For micro–computed tomography (micro-CT) analysis, 4 landmarks were chosen: (1) alveolar crest, (2) mental foramen, (3) first molar, and (4) third molar. The wild-type (WT) images in panels A and B are representative of both hard diet (HD) and soft diet (SD) since no apparent differences in phenotype were observed between these groups. (A) At 4 wk of age, micro-CT analysis indicated no striking difference in incisors of Bsp-/- mice vs. WT. (B) By 8 wk of age, micro-CT images revealed increased dentin thickness (red arrow) and decreased pulp volume toward the apical end of the incisors in Bsp-/- mice as compared with WT, regardless of diet. In addition, resorption of enamel and dentin (green arrow) and incisor-associated bone (white arrowhead) can be seen in both Bsp-/- groups. (C, E, G, I) Histology (hematoxylin and eosin) confirmed increased dentin (DE) thickness and decreased dental pulp (DP) space in Bsp-/- mouse incisors vs. WT. (D, F, H, J) Detachment (*) and disorganization of periodontal ligament (PDL) collagen fiber bundles in Bsp-/- mouse incisors with Picrosirius red staining under polarized light. Soft diet does not normalize dentinogenesis, acellular cementum formation, or PDL attachment in Bsp-/- mouse incisors.
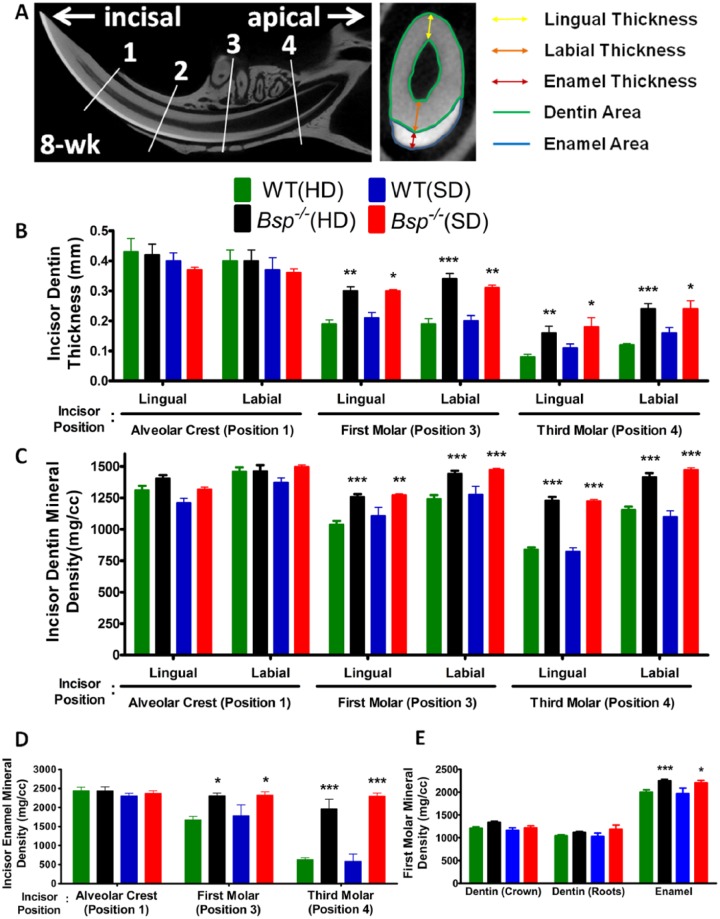
Figure 3.
Increased incisor dentinogenesis and enamel mineralization in Bsp-/- mice. (A) Based on micro–computed tomography, incisor dentin thickness, area, and mineral density were analyzed at (1) alveolar crest, (2) mental foramen, (3) first molar, and (4) third molar. Linear measurements were also performed to determine thickness of incisor enamel, labial and lingual dentin, as well as cross-sectional area of the dentin and enamel. By 8 wk of age, micro–computed tomography analysis revealed significantly increased dentin thickness (B) and mineral density (C) in the apical end of the Bsp-/- mouse incisors (incisor positions 3 and 4) when compared with those of wild type (WT), regardless of hard diet (HD) or soft diet (SD). (D) Similar to dentin, the enamel mineral density is increased significantly at incisor positions 3 and 4 of the Bsp-/- mice, regardless of diet. Interestingly, enamel and dentin mineral density at positions 3 and 4 are equivalent to the erupted incisor tissue densities (position 1) in WT and Bsp-/- mice, regardless of diet. (E) First molar crown and root dentin densities are not different in Bsp-/- vs. WT mice; however, the enamel mineral density is significantly increased in Bsp-/- molars as compared with WT, regardless of diet. For panels B to E, values shown represent means ± SEM (n = 5). *P < 0.05. **P < 0.01. ***P < 0.001. Note: Measurements of thickness and mineral density determined at positions 1 and 2 were similar to each other and showed no significant difference between WT and Bsp-/- groups; thus, only measurements at position 1 are shown.
Due to these alterations in dentinogenesis and enamel mineralization in Bsp-/- mice, irrespective of diet type, we analyzed the incisor rate of eruption. Bsp-/- mouse incisors erupted at a significantly reduced rate as compared with WT, 141 vs. 323 µm/d, respectively (protocol described in the Appendix Methods and Fig. 1). Therefore, relative alterations in dentin and enamel near incisor apices are likely due to reduced rate of eruption in Bsp-/- mice.
SD Does Not Rescue Periodontal Defects in Bsp-/- Mice
Defects noted in acellular cementum, PDL, and alveolar bone in Bsp-/- mice prompted us to determine the role of masticatory forces on these periodontal manifestations. SD did not rescue loss of acellular cementum, PDL detachment and disorganization, and resorption of incisor enamel, dentin, or associated bone in Bsp-/- mice (Figs. 2C, E, G, I). Furthermore, between 4 and 20 wk of age, Bsp-/- mice in both diet groups exhibited similar dramatic loss of alveolar bone, extensive cervical root resorption (Fig. 4A), PDL detachment, and epithelial downgrowth (Fig. 4B–I). The reduced periodontal structure contributed to premature loss of teeth, especially third molars, as the Bsp-/- mice aged.
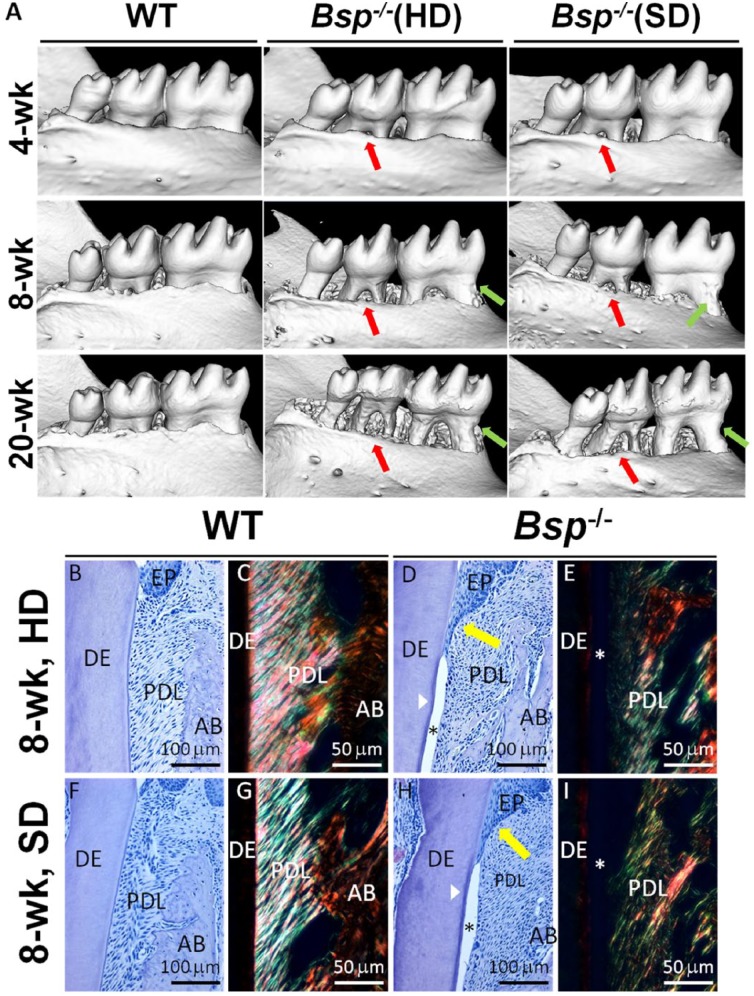
Figure 4.
Soft diet (SD) does not rescue periodontal phenotype in Bsp-/- mice. (A) Micro–computed tomography images demonstrate progressive tooth root resorption (green arrows) and alveolar bone (AB) loss (red arrows) in both Bsp-/- diet groups at 8 and 20 wk. HD, hard diet. (B, D, F, H) Hematoxylin and eosin–stained first molar sections in 8-wk-old mice reveal that Bsp-/- mice in both diet groups feature identical phenotypes, marked by lack of acellular cementum (white arrowhead), periodontal ligament (PDL) detachment (*), and epithelial (EP) downgrowth (yellow arrows); dentin (DE). (C, E, G, I) Picrosirius red–stained sections under polarized light microscopy demonstrate loss of PDL collagen organization in both Bsp-/- diet groups.
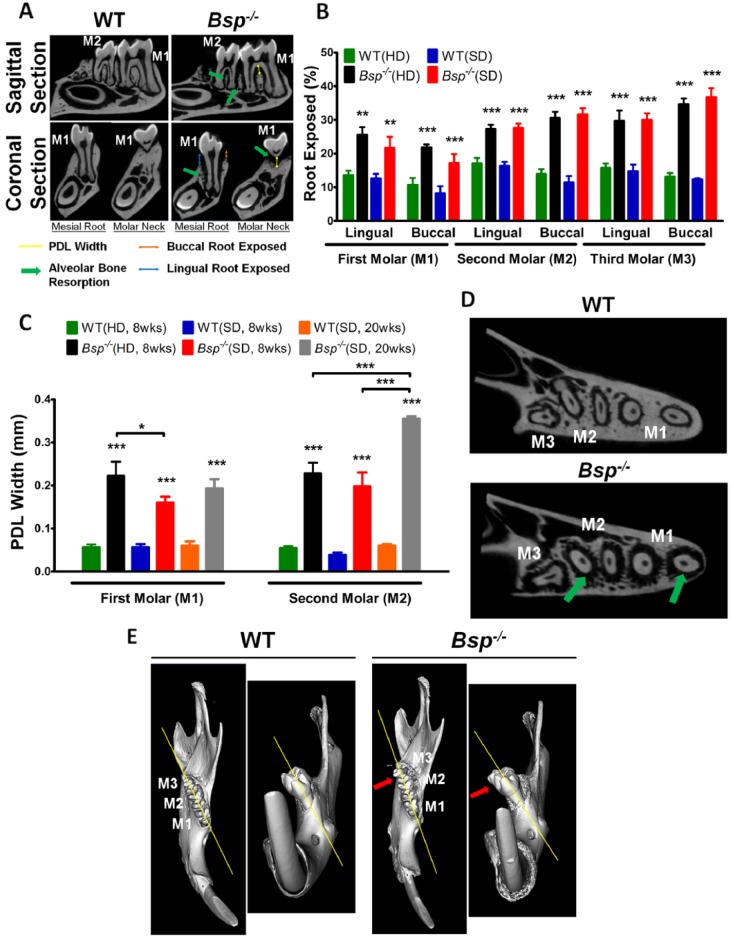
Periodontal structures were analyzed quantitatively at 8 and 20 wk, including exposure of molar roots (an indicator of alveolar bone reduction) and width of PDL in the interradicular region (Fig. 5A). Micro-CT analysis confirmed increased root exposure for all Bsp-/- molars vs. WT, regardless of diet type (Fig. 5B). At 20 wk, additional loss of alveolar bone in Bsp-/- mice was apparent, especially on the lingual side (Appendix Fig. 2). At both 8 and 20 wk, Bsp-/- mice exhibited significantly wider PDL in the interradicular regions of first and second molars (Fig. 5C), reflecting bone loss in that region as well. However, at 8 wk, Bsp-/-(SD) featured significantly less of a width increase in PDL than that of Bsp-/-(HD), indicating diet-associated amelioration of bone loss in this region. The altered periodontal structure, evidenced by reduced alveolar bone and wider PDL space (Fig. 5D), is likely responsible for the observed molar malocclusion by 8 wk of age (Fig. 5E).
Figure 5.
Progressive periodontal breakdown in mandibles of Bsp-/- mice. (A) Micro–computed tomography cross-sectional images reveal alveolar bone loss around molar roots (green arrows). For all 3 molars (M1 to M3), root exposure on buccal (blue arrow) and lingual (orange arrow) aspects and periodontal ligament (PDL) width in the interradicular region (yellow line) were determined. WT, wild type. (B) Micro–computed tomography analysis confirms significantly increased molar root exposure in all 3 molars in 8-wk-old Bsp-/- mice, with no difference in root exposure observed between both Bsp-/- diet groups. HD, hard diet; SD, soft diet. (C) At both 8 and 20 wk, Bsp-/- mice exhibited significantly wider PDL in the interradicular regions of first and second molars when compared with WT, regardless of diet. At 8 wk, Bsp-/- mice in the SD group exhibited significantly less increase in PDL width than that of the HD group, indicating diet-associated amelioration of bone loss in this region. As expected, Bsp-/-(SD) showed further widening of PDL width at 20 wk vs. 8 wk, due to progressive alveolar bone loss. At 8 wk, (D) extensive alveolar bone loss around the molar roots (green arrows) contributed to loss of periodontal support that led to (E) molar malocclusion (red arrows). For panels B and C, values represent means ± SEM (n = 5). *P < 0.05. **P < 0.01. ***P < 0.001.
Discussion
We hypothesized that the periodontal manifestations in Bsp-/- mice resulted from absence of acellular cementum, with the resulting loss of PDL attachment and associated abnormal mechanical forces leading to tooth dysfunction and periodontal tissue destruction. We tested this hypothesis using a diet designed to reduce masticatory forces, thus lowering mechanical stress on the periodontal apparatus. SD was unable to diminish periodontal breakdown associated with Bsp-/- mouse molars, including PDL detachment and disorganization, severe and progressive alveolar bone loss, and resorption of tooth root surfaces, indicating that masticatory forces are not primary factors in those pathologic changes. However, SD almost eliminated severe incisor malocclusion in Bsp-/- mice, supporting the hypothesis that occlusal loading contributed to the malocclusion phenotype. Surprisingly, SD normalized body weight, long bone length, and serum ALP activity in Bsp-/- mice, suggesting that tooth dysfunctions predispose Bsp-/- mice to feeding problems and nutritional deficits that affect body and skeletal growth and that provision of SD improves postweaning nutrition. Another surprising finding was Bsp-/- incisor phenotype of enhanced dentinogenesis and enamel mineralization that is likely due to a slower eruption rate. This study demonstrates the importance of BSP in maintaining proper periodontal function and alveolar bone remodeling, and it points to dental dysfunction as a causative factor of skeletal defects observed in Bsp-/- mice.
Role of Altered Mechanotransduction in Bsp-/- Mice Periodontal Breakdown
In this study, we demonstrated a progressive and severe alveolar bone loss in Bsp-/- mice, in contrast to reduced long-bone turnover in vivo and reduced osteoclast activation in vitro reported previously (Malaval et al. 2008). We propose 2 potential causal factors to account for the discrepancy in turnover between long bone and alveolar bone in Bsp-/- mice: excessive traumatic forces or unloading of alveolar bone resulting in altered mechanotransduction.
In normal function, alveolar bone undergoes frequent loading during mastication and is subject to continuous remodeling to accommodate tooth function (Sodek and McKee 2000). The PDL is a mechanosensitive tissue that provides connection between tooth root and supportive alveolar bone acting as a pool of progenitor cells, a source of vascularity and innervation, and, importantly, a shock absorber helping to prevent tissue damage under the forces generated by tooth use (Beertsen et al. 1997). Alveolar bone operates as a functional unit with the nonmineralized PDL, and proper mechanical and biological signals between PDL and alveolar bone are critical to maintain functional homeostasis of the periodontal apparatus (Wise and King 2008; Ho et al. 2013). Masticatory forces are transmitted through the PDL generally in an occlusal-apical direction before being distributed to the surrounding alveolar bone (Meikle 2006; Milne et al. 2009), including buccal and lingual alveolar ridges, interdental bone, and interradicular bone in multirooted teeth.
Alveolar bone resorption can result from excessive compression forces or occlusal stress by stimulation of osteoclastogenesis through local upregulation of RANKL, such as directed resorption on the compression side during orthodontic tooth movement (Walker et al. 2008; Wise and King 2008; Sanuki et al. 2010; Kook et al. 2011). Upregulation of RANKL, often associated with inflammatory-mediated bone resorption (Yoshinaga et al. 2007), was evident in the PDL region of Bsp-/-mice, although inflammatory cells were not observed (Foster et al. 2013). Furthermore, loss of PDL attachment and epithelial downgrowth in Bsp-/- mice compromise the shock-absorbing function of the PDL, disturbing the stress distribution function of the periodontium and subjecting the bony socket to increased and possibly traumatic compression forces during mastication. Extensive resorption of alveolar bone in Bsp-/- mice was most evident in the cervical root, where load-bearing horizontal and oblique PDL fibers enter bone as Sharpey fibers. In contrast, traumatic hyperocclusion in mouse molars is more associated with bone and tooth resorption near the apex and in the interradicular bone (Walker et al. 2008).
Second, alveolar bone resorption can result from absence of mechanotransduction from the PDL. Alveolar bone formation occurs as the tooth root forms and remodels in response to changes in tooth use (e.g., orthodontics) and disappears in the absence of teeth (e.g., edentulous ridges; Wise and King 2008; Hansson and Halldin 2012). These processes are regulated by the PDL, as a mechanotransductor supplying mechanical stimuli to bone and as a cellular source of biological signals including regulators of bone homeostasis (e.g., RANKL and osteoprotegerin). The functional unit of tooth, PDL, and alveolar bone can be understood through application of the mechanostat theory introduced by Harold Frost (1987), which proposes that proper functional loading is necessary to maintain structural integrity in bone through balanced resorption and formation. Similar to edentulous ridges, where lack of mechanical stimuli leads to osteopenia and alveolar ridge resorption (Hansson and Halldin 2012), Bsp-/- mice may experience alveolar bone loss as a result of disturbance of tooth attachment and reduced functional loading.
The inability of SD to ameliorate periodontal breakdown in Bsp-/- mice supports the hypothesis that loss of PDL attachment and altered mechanotransduction are contributors to reduction in alveolar bone in Bsp-/- mice. Limitations of the study include inability to measure true mechanical forces in mouse periodontia and inability to control chewing of other hard materials (e.g., cage wires, bedding) that could introduce unrecognized occlusal trauma into the SD group.
Malocclusion and Altered Incisor Formation in Bsp-/- Mice
While SD did not alter periodontal breakdown around molar teeth, it almost completely rescued the severe incisor malocclusion in Bsp-/- mice. This finding strongly supports nonphysiologic distribution of occlusal forces as a causative factor for incisor malocclusion. We also demonstrate, for the first time, evidence of molar misalignment and malocclusion in Bsp-/- mice, likely due to PDL detachment coupled with occlusal stress, similar to the incisor.
These data favor dental dysfunction and malnutrition as factors contributing to low body weight, reduced long bone length, and elevated serum ALP in Bsp-/- mice. Previous studies have demonstrated that Bsp-/- mice fed a normal hard pellet diet were smaller, with shorter long bones (Malaval et al. 2008; Bouleftour et al. 2014) and increased serum ALP (Foster et al. 2013). These parameters were normalized with provision of SD to Bsp-/- mice. Studies on periostin-null mice, which exhibit a similar periodontal breakdown and incisor malocclusion, also report similar rescue with SD in body weight and long bone length (Rios et al. 2005). Additionally, pain associated with tooth dysfunction in enamelin-null mice resulted in malnutrition and reduced body weight, which was rescued by SD (Chan et al. 2013). These parallel observations support the interpretation that postweaning malnutrition from malocclusion contributes to the bone and weight phenotype in Bsp-/- mice. However, all bone changes in Bsp-/- mice do not necessarily stem from tooth dysfunction and malnutrition. We and others have shown early delays in long-bone ossification in Bsp-/- mice (Bouleftour et al. 2014; Holm et al. 2015). Serum ALP is a commonly used biomarker for bone formation and mineralization (Yadav et al. 2012). In the Bsp-/- mice, elevation of ALP activity may serve as a compensatory mechanism for the demonstrated skeletal hypomineralization described in work by Bouleftour et al. (2014). SD helps to reduce the severe malocclusion in Bsp-/- mice, ameliorating the skeletal defect, which in turn is reflected by normalized serum ALP.
Another surprising insight is related to the rapid incisor dentin and enamel mineralization in Bsp-/- mice. Dentin is reported to include BSP (Fujisawa et al. 1993; Qin et al. 2001), yet Bsp-/- molar dentin appeared normal (Foster et al. 2013), as confirmed by micro-CT analysis in this report. Enamel is formed from dental epithelium and suspected to include BSP (Chen et al. 1998). The question arises why the incisor is so dramatically affected by loss of BSP. We determined that Bsp-/- incisors erupt at a slower rate than that of WT, causing the appearance of enhanced or accelerated dentinogenesis and enamel mineralization toward the apex. Alterations in Bsp-/- incisor eruption could result from periodontal dysfunction due to a lack of cementum and also, indirectly, chronic occlusal trauma. Previous studies indicated that migration of PDL fibroblasts contributes to the continuously erupting rodent incisor (Beertsen et al. 1997). Several other mouse models have similar incisor enamel and/or dentin phenotype, including periostin-null mice (Rios et al. 2005; Rios et al. 2008), osteoprotegerin-null mice (Sheng et al. 2010), vitamin D receptor–null mice (Zhang et al. 2009), and integrin α11β1–null mice (Popova et al. 2007). We propose that periodontal dysfunction and resulting lower rates of incisor eruption may underlie dentin and enamel changes in all of these models.
Conclusion
This study provides new insights into the role of BSP in the formation and maintenance of the periodontium, confirming the importance of BSP for acellular cementum formation that cannot be corrected by diet.
Author Contributions
Y. Soenjaya, B.L. Foster, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; F.H. Nociti Jr, contributed to data interpretation, critically revised the manuscript; M. Ao, contributed to data acquisition, critically revised the manuscript; D.W. Holdsworth, G.K. Hunter, contributed to data analysis and interpretation, critically revised the manuscript; M.J. Somerman, H.A. Goldberg, contributed to conception, design, data analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Acknowledgments
We thank Dr. Kamila Kantovitz (National Institute of Arthritis and Musculoskeletal and Skin Diseases [NIAMS] / National Institutes of Health [NIH]) for preparation of histological samples for analyses, Dr. Jane E. Aubin (University of Toronto) for providing the Bsp-null mice and for critical discussions of the work, Dr. James Simmer (University of Michigan, Ann Arbor) for insights on mouse incisor eruption, and Dr. Michael Wolf (University of Bonn, Germany) and Dr. Vivek Thumbigere-Math (NIAMS) for assistance in applying composite resin.
Footnotes
This research was supported by the Canadian Institutes for Health Research (FRN130572; H.A.G.), the Intramural Research Program of NIAMS of the NIH (M.J.S.), and NIAMS (grant AR 066110; B.L.F.). Y.S. was supported by the Joint Motion Program, a Canadian Institutes for Health Research–funded Strategic Training Initiative in Health Research and the AO Foundation (Davos, Switzerland).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Baht GS, Hunter GK, Goldberg HA. 2008. Bone sialoprotein–collagen interaction promotes hydroxyapatite nucleation. Matrix Biol. 27(7):600–608. [DOI] [PubMed] [Google Scholar]
- Beertsen W, McCulloch CA, Sodek J. 1997. The periodontal ligament: a unique, multifunctional connective tissue. Periodontol 2000. 13(1):20–40. [DOI] [PubMed] [Google Scholar]
- Boudiffa M, Wade-Gueye NM, Guignandon A, Vanden-Bossche A, Sabido O, Aubin JE, Jurdic P, Vico L, Lafage-Proust MH, Malaval L. 2010. Bone sialoprotein deficiency impairs osteoclastogenesis and mineral resorption in vitro. J Bone Miner Res. 25(12):2669–2679. [DOI] [PubMed] [Google Scholar]
- Bouleftour W, Boudiffa M, Wade-Guéye NM, Bouët G, Cardelli M, Laroche N, Vanden-Bossche A, Thomas M, Bonnelye E, Aubin JE, et al. 2014. Skeletal development of mice lacking bone sialoprotein (BSP): impairment of long bone growth and progressive establishment of high trabecular bone mass. PLoS One. 9(5):e95144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AH, Lertlam R, Simmer JP, Wang C-N, Hu JC. 2013. Bodyweight assessment of enamelin null mice. Biomed Res Int. 2013:246861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Sasaguri K, Sodek J, Aufdemorte TB, Jiang H, Thomas HF. 1998. Enamel epithelium expresses bone sialoprotein (BSP). Eur J Oral Sci. 106(S1): 331–336. [DOI] [PubMed] [Google Scholar]
- Fisher LW, Fedarko NS. 2003. Six genes expressed in bones and teeth encode the current members of the SIBLING family of proteins. Connect Tissue Res. 44(1):33–40. [PubMed] [Google Scholar]
- Foster BL. 2012. Methods for studying tooth root cementum by light microscopy. Int J Oral Sci. 4(3):119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster BL, Soenjaya Y, Nociti FH, Holm E, Zerfas PM, Wimer HF, Holdsworth DW, Aubin JE, Hunter GK, Goldberg HA, et al. 2013. Deficiency in acellular cementum and periodontal attachment in Bsp null mice. J Dent Res. 92(2):166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost HM. 1987. Bone “mass” and the “mechnostat": a proposal. Anat Rec. 219(1):1–9. [DOI] [PubMed] [Google Scholar]
- Fujisawa R, Butler WT, Brunn JC, Zhou HY, Kuboki Y. 1993. Differences in composition of cell-attachment sialoproteins between dentin and bone. J Dent Res. 72(8):1222–1226. [DOI] [PubMed] [Google Scholar]
- Ganss B, Kim RH, Sodek J. 1999. Bone sialoprotein. Crit Rev Oral Biol Med. 10(1):79–98. [DOI] [PubMed] [Google Scholar]
- Goldberg HA, Hunter GK. 2012. Functional domains of bone sialoprotein. In: Goldberg M, ed. Phosphorylated extracellular matrix proteins of bone and dentin. Vol. 2 Oak Park (IL): Bentham Science Publishers; p. 266–282. [Google Scholar]
- Goldberg HA, Warner KJ, Hunter GK. 2001. Binding of bone sialoprotein, osteopontin and synthetic polypeptides to hydroxyapatite. Connect Tissue Res. 42(1):25–37. [DOI] [PubMed] [Google Scholar]
- Hansson S, Halldin A. 2012. Alveolar ridge resorption after tooth extraction: a consequence of a fundamental principle of bone physiology. J Dent Biomech. 3:1758736012456543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SP, Kurylo MP, Grandfield K, Hurng J, Herber R-P, Ryder MI, Altoe V, Aloni S, Feng JQ, Webb S, et al. 2013. The plastic nature of the human bone–periodontal ligament–tooth fibrous joint. Bone. 57(2):455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm E, Aubin JE, Hunter GK, Beier F, Goldberg HA. 2015. Loss of bone sialoprotein leads to impaired endochondral bone development and mineralization. Bone. 71:145–154. [DOI] [PubMed] [Google Scholar]
- Hunter GK, Goldberg HA. 1993. Nucleation of hydroxyapatite by bone sialoprotein. Proc Natl Acad Sci U S A. 90(18):8562–8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kook SH, Jang YS, Lee JC. 2011. Human periodontal ligament fibroblasts stimulate osteoclastogenesis in response to compression force through TNF-α-mediated activation of CD4+ T cells. J Cell Biochem. 112(10):2891–2901. [DOI] [PubMed] [Google Scholar]
- Macneil RL, Sheng N, Strayhorn C, Fisher LW, Somerman MJ. 1994. Bone sialoprotein is localized to the root surface during cementogenesis. J Bone Miner Res. 9(10):1597–1606. [DOI] [PubMed] [Google Scholar]
- Malaval L, Monfoulet L, Fabre T, Pothuaud L, Bareille R, Miraux S, Thiaudiere E, Raffard G, Franconi JM, Lafage-Proust MH, et al. 2009. Absence of bone sialoprotein (BSP) impairs cortical defect repair in mouse long bone. Bone. 45(5):853–861. [DOI] [PubMed] [Google Scholar]
- Malaval L, Wade-Guéye NM, Boudiffa M, Fei J, Zirngibl R, Chen F, Laroche N, Roux JP, Burt-Pichat B, Duboeuf F, et al. 2008. Bone sialoprotein plays a functional role in bone formation and osteoclastogenesis. J Exp Med. 205(5):1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee MD, Zalzal S, Nanci A. 1996. Extracellular matrix in tooth cementum and mantle dentin: localization of osteopontin and other noncollagenous proteins, plasma proteins, and glycoconjugates by electron microscopy. Anat Rec. 245(2):293–312. [DOI] [PubMed] [Google Scholar]
- Meikle MC. 2006. The tissue, cellular, and molecular regulation of orthodontic tooth movement: 100 years after Carl Sandstedt. Eur J Orthod. 28(3):221–240. [DOI] [PubMed] [Google Scholar]
- Milne TJ, Ichim I, Patel B, McNaughton A, Meikle MC. 2009. Induction of osteopenia during experimental tooth movement in the rat: alveolar bone remodelling and the mechanostat theory. Eur J Orthod. 31(3):221–231. [DOI] [PubMed] [Google Scholar]
- Popova SN, Barczyk M, Tiger C-F, Beertsen W, Zigrino P, Aszodi A, Miosge N, Forsberg E, Gullberg D. 2007. α11β1 integrin-dependent regulation of periodontal ligament function in the erupting mouse incisor. Mol Cell Biol. 27(12):4306–4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, Brunn JC, Jones J, George A, Ramachandran A, Gorski JP, Butler WT. 2001. A comparative study of sialic acid-rich proteins in rat bone and dentin. Eur J Oral Sci. 109(2):133–141. [DOI] [PubMed] [Google Scholar]
- Rios H, Koushik SV, Wang H, Wang J, Zhou HM, Lindsley A, Rogers R, Chen Z, Maeda M, Kruzynska-Frejtag A, et al. 2005. Periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol Cell Biol. 25(24):11131–11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios HF, Ma D, Xie Y, Giannobile WV, Bonewald LF, Conway SJ, Feng JQ. 2008. Periostin is essential for the integrity and function of the periodontal ligament during occlusal loading in mice. J Periodontol. 79(8):1480–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanuki R, Shionome C, Kuwabara A, Mitsui N, Koyama Y, Suzuki N, Zhang F, Shimizu N, Maeno M. 2010. Compressive force induces osteoclast differentiation via prostaglandin E2 production in MC3T3-E1 cells. Connect Tissue Res. 51(2):150–158. [DOI] [PubMed] [Google Scholar]
- Sheng ZF, Ye W, Wang J, Li CH, Liu JH, Liang QC, Li S, Xu K, Liao EY. 2010. OPG knockout mouse teeth display reduced alveolar bone mass and hypermineralization in enamel and dentin. Arch Oral Biol. 55(4):288–293. [DOI] [PubMed] [Google Scholar]
- Sodek J, McKee MD. 2000. Molecular and cellular biology of alveolar bone. Periodontol 2000. 24(1):99–126. [DOI] [PubMed] [Google Scholar]
- Tye CE, Hunter GK, Goldberg HA. 2005. Identification of the type I collagen-binding domain of bone sialoprotein and characterization of the mechanism of interaction. J Biol Chem. 280(14):13487–13492. [DOI] [PubMed] [Google Scholar]
- Walker CG, Ito Y, Dangaria S, Luan X, Diekwisch TG. 2008. RANKL, osteopontin, and osteoclast homeostasis in a hyperocclusion mouse model. Eur J Oral Sci. 116(4):312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise GE, King GJ. 2008. Mechanisms of tooth eruption and orthodontic tooth movement. J Dent Res. 87(5):414–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav MC, de Oliveira RC, Foster BL, Fong H, Cory E, Narisawa S, Sah RL, Somerman M, Whyte MP, Millán JL. 2012. Enzyme replacement prevents enamel defects in hypophosphatasia mice. J Bone Miner Res. 27(8):1722–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga Y, Ukai T, Abe Y, Hara Y. 2007. Expression of receptor activator of nuclear factor kappa B ligand relates to inflammatory bone resorption, with or without occlusal trauma, in rats. J Periodontal Res. 42(5):402–409. [DOI] [PubMed] [Google Scholar]
- Zhang X, Rahemtulla F, Zhang P, Li X, Beck P, Thomas HF. 2009. Normalisation of calcium status reverses the phenotype in dentin, but not in enamel of VDR-deficient mice. Arch Oral Biol. 54(12):1105–1110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.