Abstract
Alendronate (ALN) is an antiresorptive agent widely used for the treatment of osteoporosis. Its suppressive effect on osteoclasts has been extensively studied. However, the effect of ALN on bone formation is not as clear as its effect on resorption. The objective was to determine the effect of short-term ALN on bone formation and tooth extraction wound healing. Molar tooth extractions were performed in mice. ALN, parathyroid hormone (PTH), or saline (vehicle control) was administered. PTH was used as the bone anabolic control. Mice were euthanized at 3, 5, 7, 10, and 21 d after extractions. Hard tissue healing was determined histomorphometrically. Neutrophils and lymphatic and blood vessels were quantified to evaluate soft tissue healing. Gene expression in the wounds was assessed at the RNA level. Furthermore, the vossicle bone transplant system was used to verify findings from extraction wound analysis. Alkaline phosphatase (ALP) was visualized in the vossicles to assess osteoblast activity. ALN exhibited no negative effect on bone formation. In intact tibiae, ALN increased bone mass significantly more than PTH did. Consistently, significantly elevated osteoblast numbers were noted. In the extraction sockets, bone fill in the ALN-treated mice was equivalent to the control. Genes associated with bone morphogenetic protein signaling, such as bmp2, nog, and dlx5, were activated in the extraction wounds of the ALN-treated animals. Bone formation in vossicles was significantly enhanced in the ALN versus PTH group. In agreement with this, ALN upregulated ALP activity considerably in vossicles. Neutrophil aggregation and suppressed lymphangiogenesis were evident in the soft tissue at 21 d after extraction, although gross healing of extraction wounds was uneventful. Bone formation was not impeded by short-term ALN treatment. Rather, short-term ALN treatment enhanced bone formation. ALN did not alter bone fill in extraction sockets.
Keywords: diphosphonates, osteoclasts, osteoblasts, neutrophils, inflammation, lymphatic vessels
Introduction
Alendronate (ALN) is a bisphosphonate widely used for the treatment of osteoporosis. ALN suppresses bone resorption, thereby preventing bone fractures. The mechanism of ALN to suppress bone resorption has been extensively studied (Luckman et al. 1998). However, the effect of ALN on bone formation is not as clear as its effect on resorption. It has been reported that ALN had a cytotoxic effect on cells in the osteoblast lineage (Idris et al. 2008) and suppressed bone formation in rat long bones (Iwata et al. 2006). In a case report, reduced bone formation was observed in patients with arthritis treated with ALN (Somford et al. 2009). The report, however, failed to determine whether suppressed bone formation was attributed to debilitated osteoblast function alone or perhaps as a coupling effect with suppressed bone resorption by ALN. Contrarily, there is evidence that ALN exerts a bone anabolic effect. ALN was found to stimulate osteogenic differentiation of mesenchymal cells and mineral nodule formation ex vivo (Duque and Rivas 2007; Lindtner et al. 2014). ALN promoted the repair of critical-sized calvarial defects by enhancing bone formation (Wang et al. 2010; Toker et al. 2012). In a clinical trial where periodontal defects were treated with ALN gel, positive outcomes were obtained in bone fill (Sharma and Pradeep 2012). Taken together, whether ALN has a positive or negative impact on bone formation in vivo, independently of resorption, remains elusive.
Tooth extractions involve tissue injuries in which soft tissue laceration and bone exposure to the oral environment are inevitable. Inflammation occurs immediately after extractions in response to trauma and bacterial insults. Osteoclasts emerge in the crestal side of sockets to resorb damaged bone (Pietrokovski and Massler 1971). As inflammation subsides, woven bone formation increases. Osteoblasts are predominant cells in this bone formation phase (Guglielmotti and Cabrini 1985). Woven bone matures and is subject to remodeling, which occurs 6 to 12 mo after extraction(s) in humans (Trombelli et al. 2008). Thus, bone formation and the resolution of inflammation are important events of early soft tissue and extraction socket healing. The effect of ALN on tooth extraction wound healing has been studied in relation to osteonecrosis of the jaw (ONJ). It is generally agreed that ALN monotherapy does not induce ONJ-like impaired healing (Aguirre et al. 2010). Bone fill, in extraction sockets, is not compromised by ALN (Kim et al. 2011). Furthermore, ALN treatment maintained the ridge height better than natural healing after tooth extractions (Jee et al. 2010). These findings imply that ALN may have beneficial effects on tooth extraction wound healing. The current study aimed to delineate the effect of short-term ALN monotherapy not only on tooth extraction wound healing but also on bone formation to determine whether ALN has bone anabolic effects. Intermittent parathyroid hormone (PTH) administration, a well-documented bone anabolic treatment (Kuroshima et al. 2013), was also performed as a comparison to ALN treatment.
Materials and Methods
Animals, Tooth Extractions, Injections, and Vossicle Model
C57BL/6J male mice (n = 105) were randomly divided into 15 groups (n = 7/group). At the age of 8 wk, the maxillary right first molars were extracted. Either ALN (100 μg/kg/d) or PTH (40 μg/kg/d) was administered subcutaneously for 3, 5, 7, 10, and 21 d after extractions. Saline was used as the vehicle control (VC). Another 36 mice underwent tooth extractions and received subsequent injections for 5, 10, and 21 d in the same manner as described above. The tissues at the extraction sites were harvested at sacrifice and used for gene expression analysis.
The fresh vertebrae from neonatal mice were harvested and subcutaneously implanted in the dorsal surface of adult mice as described previously (Yamashita et al. 2008). ALN (100 μg/kg/d), PTH (40 μg/kg/d), or saline was administered daily for 10 d. At 10 d, the implanted vertebrae (vossicles) were removed (Appendix Fig.). The experimental protocol was approved by the University Committee on Use and Care of Animals.
Histology and Immunohistochemistry
The maxillae, tibiae, and vossicles were dissected, fixed, and decalcified. Hematoxylin and eosin staining, tartrate-resistant acid phosphatase staining, and Masson’s trichrome staining were performed. Immunofluorescent staining of lymphatic and blood vessels and neutrophils was performed. Lymphatic endothelial hyaluronan receptor-1 (LYVE-1), CD31, and Ly6G antibodies were used. DAPI was used for counterstaining. Alkaline phosphatase (ALP) staining was performed on vossicle sections to detect osteogenic activity (Miao and Scutt 2002).
Histomorphometric Analysis
Stained sections were histomorphometrically analyzed. Collagen, new bone, blood and lymphatic vessels, osteoclasts, Ly6G+ cells, and empty osteocyte lacunae were assessed. In addition, ALP activity was assessed in the vossicles.
Gene Expression Assessment
The tissues at the extraction sites were harvested and processed for RNA isolation. Total RNA was analyzed using the Mouse Osteogenesis PCR Array (Qiagen, Valencia, CA, USA).
Serum Chemistry and Complete Blood Count (CBC)
Blood was collected at sacrifice and CBC performed. Serum tartrate–resistant acid phosphatase 5b (TRAcP5b) and procollagen type I N-terminal propeptide (P1NP) were also measured.
Statistics
Analysis of variance with a Tukey test and Fisher exact test were performed. An α level of 0.05 was used for statistical significance. Results are presented as mean ± standard error unless otherwise specified. Detailed descriptions of materials and methods are provided in the Appendix Materials and Methods section.
Results
ALN Did Not Negatively Impact Bone Fill
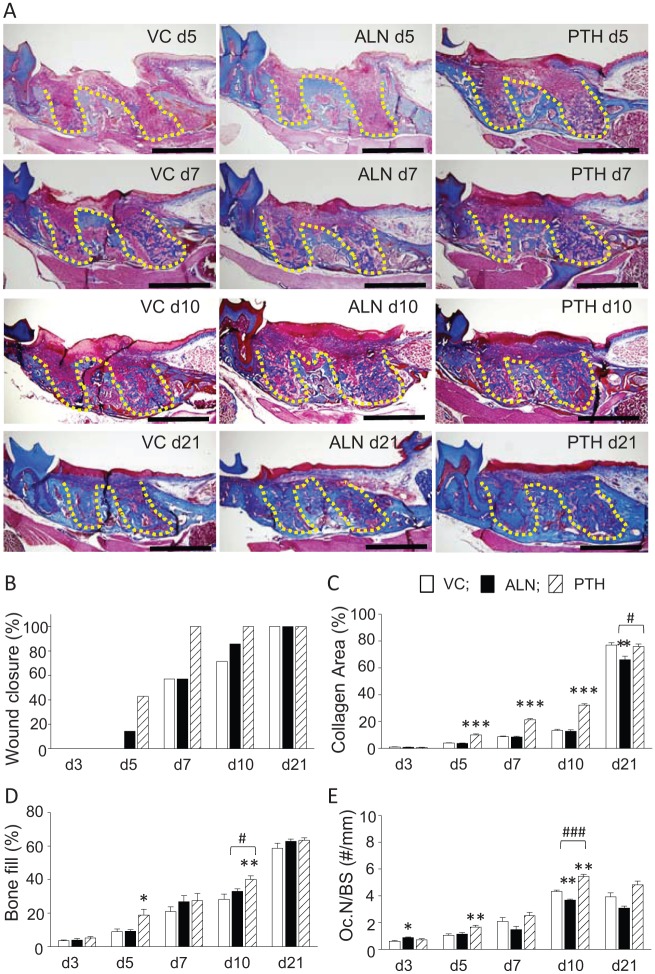
Representative photomicrographs of trichrome-stained sections of the extraction wounds are shown in Figure 1A. Wound closure was indicated by the observance of epithelial coverage. No epithelial coverage was noted at 3 d. Epithelialization was seldom observed in the ALN and control groups at 5 d (Fig. 1B). Epithelial coverage was seen in all wounds in the PTH group at 7 d, whereas coverage was found in approximately half of the wounds in the ALN and control groups. At 10 d, epithelial coverage was still not found in all the wounds in the ALN and control groups; however, all wounds, irrespective of treatment, were covered by epithelium at 21 d. No differences were noted in collagen apposition between the ALN and control groups at 3, 5, 7, and 10 d. At 21 d, however, the ALN group showed significantly less collagen apposition than the control (Fig. 1C). On the other hand, PTH treatment significantly promoted collagen apposition at 5, 7, and 10 d compared with the control. At 21 d, however, such a difference was no longer detected. Bone fill increased with time in all groups (Fig. 1D). There was a trend of greater bone fill in the ALN group than the control at 7, 10, and 21 d. PTH treatment significantly enhanced bone fill compared with the control at 5 and 10 d. At 21 d, bone fill was similar between the ALN and PTH groups. Thus, at 3 wk after extractions, no differences were observed in wound closure and bone fill between groups, but collagen apposition was diminished in the ALN group versus the control and PTH groups. Osteoclast numbers per bone perimeter in the extraction wounds increased until 10 d and then declined at 21 d regardless of treatment (Fig. 1E). A lower osteoclast number was observed in the ALN group versus the control, but a statistical difference was achieved only at 10 d. PTH significantly increased the osteoclast number at 5 and 10 d compared with the control. At 21 d, the osteoclast number was similar between the PTH and control groups.
Figure 1.
Parathyroid hormone (PTH) promoted while alendronate (ALN) had no negative effect on bone fill in tooth extraction sockets. (A) Representative photomicrographs of trichrome-stained sections of tooth extraction wounds at 5 (top), 7 (second row), 10 (third row), and 21 (bottom) d after extractions. Gross healing was uneventful regardless of treatment. The yellow dotted line depicts tooth extraction sockets. Scale bar: 1 mm. (B) Epithelium coverage was promoted in the PTH group, while coverage in the ALN group was similar to the vehicle control (VC). However, the Fisher exact test revealed no statistically significant difference between groups (P = 0.09 at 5 d and P = 0.07 at 7 d). (C) PTH promoted collagen apposition in the connective tissue, while ALN had no effect except at 21 d when there was significantly less collagen area noted in the ALN group versus the others. (D) PTH promoted while ALN showed no effect on bone fill. At 21 d, no differences were noted in bone fill between groups. (E) Osteoclasts were significantly promoted in the PTH group at 5 and 10 d. ALN generally suppressed osteoclasts after 7 d. However, significant suppression was noted at 10 d only. n = 7/group. *P < 0.05, **P < 0.01, and ***P < 0.001 versus VC. #P < 0.05 and ###P < 0.001 versus PTH.
ALN Suppressed Lymphangiogenesis
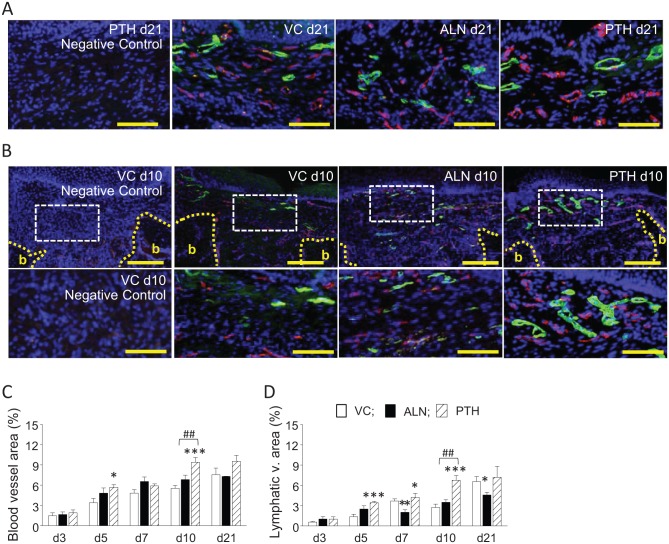
Blood and lymphatic vessel formation was assessed to evaluate soft tissue healing. No significant differences were noted in the blood vessel area between the ALN and control groups at any day examined (Fig. 2A, C). However, the lymphatic vessel area in the ALN group was significantly smaller than the control at 7 and 21 d (Fig. 2B, D). On the other hand, PTH was hemoangiogenic and lymphangiogenic. PTH significantly increased the blood vessel area at 5 and 10 d and the lymphatic vessel area at 5, 7, and 10 d compared with the control. Thus, ALN suppressed while PTH promoted lymphangiogenesis (Fig. 2C, D).
Figure 2.
Parathyroid hormone (PTH) promoted both blood and lymphatic vessel formation. Alendronate (ALN) had no effect on blood vessels but suppressed lymphatic vessel formation. (A) Representative photomicrographs of extraction wound sections at 21 d. The soft tissue areas in the wounds are shown. Blood and lymphatic vessels were immunofluorescently labeled with CD31 (pink) and LYVE-1 (green), respectively. DAPI (blue) counterstaining was used to visualize nuclei. Negative control (far left): Isotype controls were used instead of primary antibodies. Scale bar: 100 µm. (B) Representative photomicrographs of extraction wound sections at 10 d. The soft tissue areas above the extraction sockets are shown (top). b, bone. Scale bar: 200 µm. Images below are magnified views of the dotted white rectangles. Large blood and lymphatic vessels are noted in the PTH group versus the others. Negative control (far left): Isotype controls were used instead of primary antibodies. Scale bar: 100 µm. (C) ALN had no negative effect on the blood vessel area, while PTH significantly promoted blood vessels at 5 and 10 d. (D) A significantly larger lymphatic vessel area was noted at 5, 7, and 10 d in the PTH group, while a significantly smaller lymphatic vessel area was noted at 5 and 21 d in the ALN group. n = 7/group. *P < 0.05, **P < 0.01, and ***P < 0.001 versus vehicle control (VC). ##P < 0.01 versus PTH.
ALN Retained Neutrophils
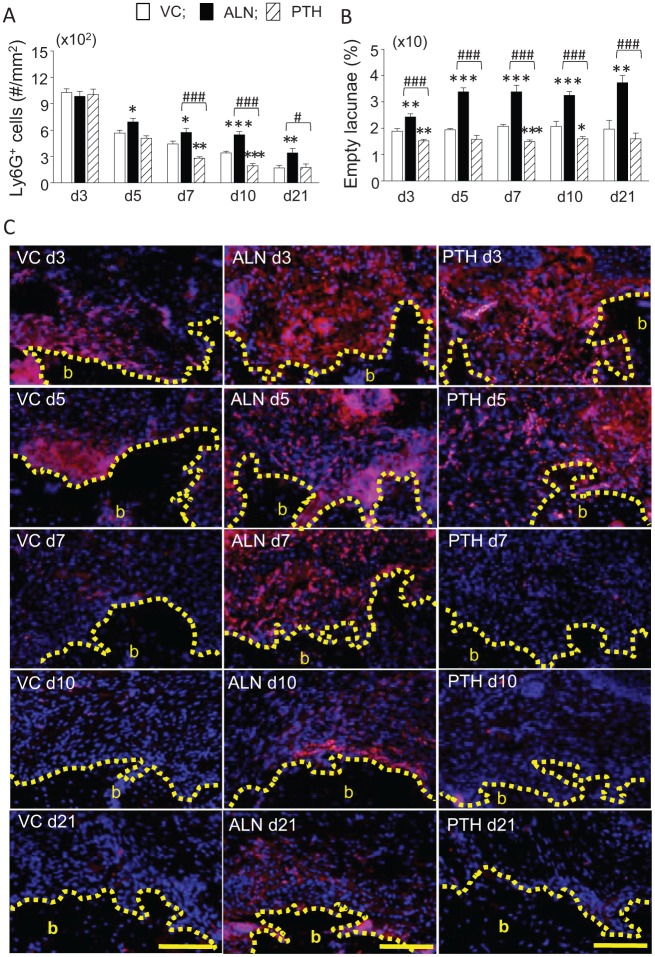
Neutrophils were assessed to examine inflammation in the soft tissue. At 3 d, intense neutrophil infiltration (Ly6G+) was high and similar in all groups (Fig. 3A). This intense neutrophil infiltration was resolved over time in all groups; however, significantly reduced numbers of neutrophils were noted in the PTH group versus the control at 7 and 10 d. On the other hand, resolution occurred at a much slower pace in the ALN group versus the control at all time points after 3 d. At 21 d, the number of neutrophils in the ALN group was still significantly higher than the other groups. In the ALN group, congregated neutrophils were retained near the alveolar crest 3 wk after extractions (Fig. 3C). The numbers of empty osteocyte lacunae in the crestal bone were evaluated to explore a mechanism of the large neutrophil retention (Fig. 3B). The average percentage of empty lacunae was approximately 2% in the control, while in the ALN group, it was nearly doubled when compared with the control. Significantly less empty lacunae were observed in the PTH group versus the control at 3, 7, and 10 d. In peripheral blood at 5 d, significant increases in red blood cell and platelet counts were noted in the PTH group versus the control (Appendix Table 1). ALN significantly increased the hemoglobin and hematocrit values at 5 d. However, no differences were noted in the numbers of neutrophils and lymphocytes between groups at 5, 10, and 21 d (Fig. 4A).
Figure 3.
There were increased numbers of neutrophils and empty osteocyte lacunae found with alendronate (ALN) treatment. (A) Significantly larger numbers of neutrophils were retained in the healing soft tissue of wounds with ALN treatment through 21 d. Conversely, parathyroid hormone (PTH) resolved neutrophil aggregation significantly at 7 and 10 d. (B) Continuously retained empty lacunae were noted in the ALN group, while PTH reduced the numbers of empty lacunae at 3, 7, and 10 d. (C) Representative photomicrographs of extraction wound sections at 3, 5, 7, 10, and 21 d. The soft tissue areas in the wounds are shown. Neutrophils were immunofluorescently labeled with Ly6G (pink). Nuclear DAPI (blue) counterstaining was used. An increased neutrophil presence was detected near the bone surface in the ALN group (middle). b, bone. Scale bar: 100 µm. n = 7/group. *P < 0.05, **P < 0.01, and ***P < 0.001 versus vehicle control (VC). #P < 0.05 and ###P < 0.001 versus PTH.
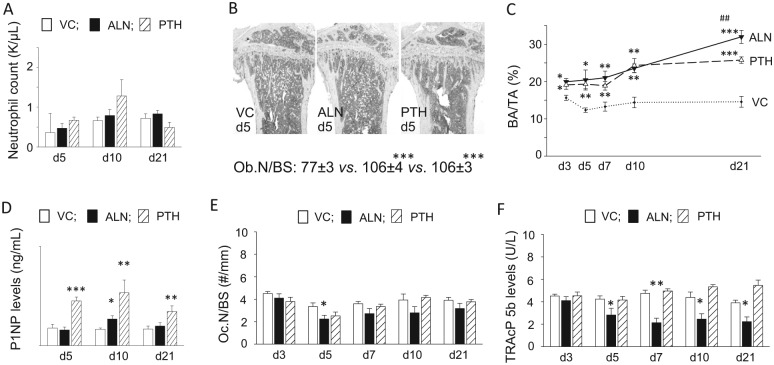
Figure 4.
Parathyroid hormone (PTH) and alendronate (ALN) treatment increased bone area in long bone. (A) Neutrophil numbers in peripheral blood at 5, 10, and 21 d. No differences were found between groups. (B) Representative photomicrographs of tartrate-resistant acid phosphatase–stained sections of proximal tibiae at 5 d (original magnification, ×40). Osteoblast numbers per linear bone perimeter were enumerated in hematoxylin and eosin–stained sections. Significantly higher numbers of osteoblasts per bone surface were noted in the ALN and PTH groups compared with the vehicle control (VC). (C) The bone area increased in the ALN and PTH groups over 21 d of treatment as compared with the VC. At 21 d, bone mass was significantly higher in the ALN group when compared with the PTH group. (D) The average serum P1NP levels were plotted at 5, 10, and 21 d. A significantly higher P1NP level was noted at 10 d in the ALN group versus the VC. In the PTH group, the serum P1NP levels were constantly significantly higher than those in the VC. (E) No significant suppression in osteoclast numbers per bone perimeter was found in the ALN group except at 5 d. PTH treatment did not affect the osteoclast number. However, the average serum TRAcP5b levels were consistently significantly lower in the ALN group than the VC (F). n = 7/group. *P < 0.05, **P < 0.01, and ***P < 0.001 versus VC. ##P < 0.01 versus PTH.
ALN Increased Bone Mass in Long Bone Comparable with PTH
ALN and PTH significantly increased bone area in the proximal tibiae compared with the control (Fig. 4B, C). The osteoblast number at 5 d was significantly higher in the ALN and PTH groups than the control. In the PTH group, bone area greatly increased from 7 to 10 d and then plateaued thereafter, while bone area steadily expanded through 21 d in the ALN group. At 21 d, average bone mass was significantly higher in the ALN group than the PTH group. The serum P1NP level, a marker for bone formation (Chen et al. 2005), was significantly higher in the PTH group versus the VC at 5, 10, and 21 d (Fig. 4D). However, ALN significantly increased the P1NP level only at 10 d. While ALN significantly decreased the osteoclast number per bone surface at 5 d, a trend was observed at all subsequent time points (Fig. 4E). Results of the serum TRAcP5b measurement, however, revealed that ALN significantly suppressed osteoclasts at 5, 7, 10, and 21 d (Fig. 4F).
ALN Activated Bone Morphogenetic Protein (BMP) Signaling
Treatment effects on osteogenesis-related gene expression in the extraction wounds were assessed at 5, 10, and 21 d. The upregulation of bmp2 and twist1 at 21 d as compared with 5 d was noted in all groups (Appendix Table 2). In the ALN group, genes associated with BMP2 signaling, such as bmp2, acvr1, bmpr2, chrd, nog, smd3, and sost, were upregulated at 21 d versus 5 d. ALN upregulated vegfa and cdh11, which are associated with angiogenesis and osteoblast adhesion, respectively.
ALN Stimulated ALP Activity in Vossicles
The vossicle model was employed to determine the effect of ALN on bone formation. ALN and PTH significantly promoted bone growth in vossicles compared with the control, with significantly more bone in the ALN group than the PTH group (Fig. 5A, D). Consistently, ALP activity was significantly higher in the ALN and PTH groups than the control but not different from each other (Fig. 5B, E). Osteoclast numbers were significantly lower in the ALN group than in the control and PTH groups (Fig. 5C, F).
Figure 5.
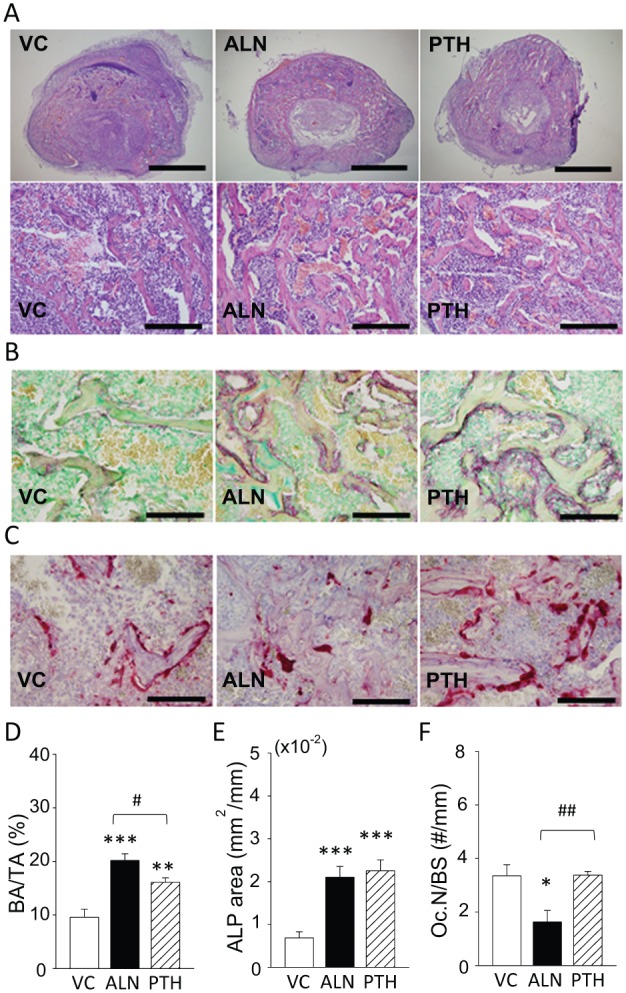
Alendronate (ALN) treatment stimulated alkaline phosphatase (ALP) activity in ectopically implanted bone. (A) Fresh vertebrae were harvested from neonatal mice and subcutaneously implanted in young adult mice. The recipient mice received ALN, parathyroid hormone (PTH), or vehicle control (VC) treatment for 10 d. Representative photomicrographs of hematoxylin and eosin–stained vossicles are shown (top row, scale bar: 1 mm) and images with higher magnification (bottom row, scale bar: 200 µm). (B) ALP was visualized in vossicles. The red color indicates an ALP-positive area. An ALP-positive area was found on the bone surface where osteoblasts attached. Scale bar: 100 µm. (C) Representative photomicrographs of tartrate-resistant acid phosphatase–stained sections. Scale bar: 100 µm. (D) ALN promoted bone growth significantly more compared with PTH. (E) Both ALN and PTH significantly stimulated ALP activity in vossicles. (F) ALN significantly suppressed osteoclasts compared with others. n = 7/group. *P < 0.05, **P < 0.01, and ***P < 0.001 versus VC. #P < 0.05 and ##P < 0.01 versus PTH.
Discussion
These findings signify that ALN had no negative impact on bone formation in vivo. ALN had no effect on bone fill in a wound-healing scenario; however, it promoted bone apposition in young adult mice and in a bony implant model. In a tooth extraction wound-healing scenario, osteoclasts emerge around the bony crest to resorb damaged bone (Guglielmotti and Cabrini 1985). Bone formation predominantly occurs from the apical half side and projects into the entire socket (Smith 1974). In this study, bone fill increased steadily as healing time increased to 21 d in all groups. No deterred bone fill was observed in the ALN group at any day compared with the VC. Therefore, it is clear that ALN exhibited no negative impact on bone fill. In support of this, the expression of osteogenic genes was observed in all groups at 21 d versus 5 d, indicating ongoing bone formation. The net bone mass reflects a balance between bone formation and resorption. Osteoclast suppression typically leads to an increase in bone mass. To gain insight into the balance in healing extraction sockets, osteoclasts on the bone surface were enumerated. A trend of decreased osteoclast numbers was observed in the ALN group, but statistical differences were not reached except at 10 d. Hence, there were ample osteoclasts attached to the bone surface in healing sockets in all groups. It is not uncommon to observe numerous osteoclasts attached to bone in healing sockets of bisphosphonate-treated animals. Kim et al. (2011) investigated the ALN effect on healing at 3, 7, 14, and 28 d after tooth extractions and found no differences in osteoclast numbers between ALN-treated and control mice. Since ALN is a potent bisphosphonate, it should suppress osteoclasts on the bone surface. However, this was not the case in tooth extraction wounds as osteoclasts were not greatly suppressed. While exact mechanisms for this are unknown, it is plausible to consider that high bone metabolism in healing sockets likely generated robust biological cues to induce osteoclastogenesis, and because of rapid bone apposition, the bone surface to which ALN originally adhered was rapidly covered with a new bone layer, presenting a surface to which osteoclasts could attach and not be harmed. ALN lowered osteoclast numbers in the tibiae. Serum TRAcP5b levels were significantly lower in the ALN group. Therefore, the expected osteoclast suppressive effect of ALN was indeed observed. On the other hand, ALN and PTH significantly increased bone mass throughout the experimental period, indicating that both treatments achieved their therapeutic goal, which is to increase bone mass. Consistently, osteoblast numbers in the ALN group were comparable with those in the PTH group and significantly higher than the control. The elevated osteoblast numbers in the ALN group suggest that osteoblast proliferation was stimulated.
It is a challenge to determine whether ALN promotes bone formation independent of bone resorption in vivo. This is because suppressed osteoclasts may directly or indirectly influence bone formation. To verify that ALN promoted osteoblasts in vivo, we employed the vossicle bone transplant system (Pettway and McCauley 2008) and assessed ALP activity. As expected, both ALN and PTH significantly promoted vossicle growth, with significantly more bone mass in the ALN group than the PTH group; indeed, bone mass in the ALN group was nearly doubled compared with the VC. It is prudent to reason that osteoclast suppression by ALN contributed to the increased bone mass. However, considering that ALN promoted bone fill in extraction sockets where numerous osteoclasts attached, and that ALN stimulated BMP signaling in the extraction wounds, we speculated that ALN promoted osteoblasts in addition to osteoclast suppression in vossicles. To assess osteoblast activity, ALP was quantified. It was found that both ALN and PTH significantly stimulated ALP expression in the vossicles. Since ALP reflects matrix mineralization (Anderson 1995), our finding indicates that ALN promoted osteoblastic bone formation similarly to PTH. It should be mentioned that the findings from the vossicle implant system may not entirely hold true in the extraction wound-healing scenario. In extraction wounds, where the repair/modeling of hard and soft tissues takes place, immune responses to bacterial insults and mechanical trauma are early major events during healing. On the other hand, undisturbed bone growth is the main event in the vossicle transplant system. Thus, the biological environment is distinct between extraction wounds and vossicle transplants. However, since the vossicle environment is free from microflora, trauma, and soft tissue formation, bone metabolic responses to ALN treatment were more focally characterized with this system, and therefore, the findings from the vossicle system would reflect a basic bone response to ALN.
Lymphatic and blood vessels are essential components of soft tissue. In this study, lymphatic and blood vessels were assessed in the soft tissue healing context. PTH promoted lymphatic and blood vessel formation, while ALN had no effect on blood vessels but suppressed lymphangiogenesis. The role of lymphatic vessels in oral wounds is mostly unknown; however, taking that lymphatic drainage suppresses edema and facilitates immune cell trafficking (Berggreen and Wiig 2013), suppressed lymphangiogenesis in the ALN group suggests that soft tissue healing was hindered. Consistently, PTH enhanced wound closure and collagen apposition. ALN had no effect on wound closure but suppressed collagen apposition at 21 d. Based on these observations, we surmised that PTH enhanced while ALN hindered soft tissue healing. It has been reported that zoledronate and pamidronate reduced circulating neutrophils and impeded their chemotaxis (Kuiper et al. 2012). To determine the systemic effect of ALN on neutrophils in this study, neutrophil numbers in blood were counted and determined to be no different from vehicle-treated mice. To elucidate a mechanism of blunt extraction wound healing in the ALN group, inflammation was immunofluorescently examined by quantifying neutrophils. As anticipated, neutrophils decreased constantly as healing time increased in the PTH and control groups. However, in the ALN group, neutrophils did not decrease in the manner observed in the control or PTH groups. In fact, significant numbers of neutrophils were retained throughout the experimental period. Thus, inflammation was sustained and therefore soft tissue healing delayed in the ALN group. A firm association was recognized between empty lacunae and neutrophil aggregation. Intense neutrophil aggregation was predominantly found alongside crestal bone. With ALN treatment, the resultant number of empty osteocyte lacunae was significantly increased and found in the crestal bone area next to tissue with increased neutrophil aggregation. Since trauma causes cell damage and triggers an influx of neutrophils (Iyer et al. 2009), a damaged bone portion devoid of viable osteocytes seemingly provoked neutrophil aggregation. Thus, sustained inflammation in the wounds was attributed to the retained damaged bone in the ALN-treated animals. The retention of damaged bone with empty lacunae in the alveolar crest of bisphosphonate-treated animals is a common finding in the literature (Abtahi et al. 2012; Kuroshima et al. 2014). Different from inside the extraction sockets, the crestal bone area is subject to bone resorption, and minimal bone apposition is expected after extractions (Araujo and Lindhe 2005). This would result in continuous ALN accumulation on the bony crest, which slows local bone resorption, thereby causing the retention of empty lacunae. In fact, retention of the ridge height by bisphosphonates has been reported in the literature (Jee et al. 2010). Thus, sustained inflammation in connective tissue in the ALN group was due to the suspended resorption of damaged bone. Clinically, this finding may suggest that efforts to minimize bone damage during tooth extractions are important for the advancement of wound healing when ALN is used. It should be mentioned that short-term ALN administration was initiated at the time of tooth extractions in bisphosphonate-naive mice in this study. Therefore, the healing pattern could be different when tooth extractions are performed in mice on previous long-term bisphosphonate treatment.
In summary, ALN monotherapy did not negatively affect bone formation in the context of tooth extraction wound healing and in fact promoted bone formation in the vossicle implant model. Although gross healing of tooth extraction wounds was uneventful, ALN delayed the resolution of neutrophil aggregation in connective tissue. Sustained inflammation was seemingly attributed to the suspended resorption of damaged bone by ALN. This study shows that short-term ALN administration following tooth extractions has the potential to enhance bone fill in the extraction sockets.
Author Contributions
R. Tanoue, contributed to conception, data acquisition, analysis, and interpretation, drafted the manuscript; K. Koi, contributed to conception, data acquisition, and analysis, drafted the manuscript; J. Yamashita, contributed to conception, design, data acquisition, analysis, and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This work was supported by the National Institutes of Health/National Institute of Dental and Craniofacial Research R01DE 023538.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Abtahi J, Agholme F, Sandberg O, Aspenberg P. 2012. Bisphosphonate-induced osteonecrosis of the jaw in a rat model arises first after the bone has become exposed: no primary necrosis in unexposed bone. J Oral Pathol Med. 41(6):494–499. [DOI] [PubMed] [Google Scholar]
- Aguirre JI, Altman MK, Vanegas SM, Franz SE, Bassit AC, Wronski TJ. 2010. Effects of alendronate on bone healing after tooth extraction in rats. Oral Dis. 16(7):674–685. [DOI] [PubMed] [Google Scholar]
- Anderson HC. 1995. Molecular biology of matrix vesicles. Clin Orthop Relat Res. 314:266–280. [PubMed] [Google Scholar]
- Araujo MG, Lindhe J. 2005. Dimensional ridge alterations following tooth extraction: an experimental study in the dog. J Clin Periodontol. 32(2):212–218. [DOI] [PubMed] [Google Scholar]
- Berggreen E, Wiig H. 2013. Lymphangiogenesis and lymphatic function in periodontal disease. J Dent Res. 92(12):1074–1080. [DOI] [PubMed] [Google Scholar]
- Chen P, Satterwhite JH, Licata AA, Lewiecki EM, Sipos AA, Misurski DM, Wagman RB. 2005. Early changes in biochemical markers of bone formation predict BMD response to teriparatide in postmenopausal women with osteoporosis. J Bone Miner Res. 20(6):962–970. [DOI] [PubMed] [Google Scholar]
- Duque G, Rivas D. 2007. Alendronate has an anabolic effect on bone through the differentiation of mesenchymal stem cells. J Bone Miner Res. 22(10):1603–1611. [DOI] [PubMed] [Google Scholar]
- Guglielmotti MB, Cabrini RL. 1985. Alveolar wound healing and ridge remodeling after tooth extraction in the rat: a histologic, radiographic, and histometric study. J Oral Maxillofac Surg. 43(5):359–364. [DOI] [PubMed] [Google Scholar]
- Idris AI, Rojas J, Greig IR, Van’t Hof RJ, Ralston SH. 2008. Aminobisphosphonates cause osteoblast apoptosis and inhibit bone nodule formation in vitro. Calcif Tissue Int. 82(3):191–201. [DOI] [PubMed] [Google Scholar]
- Iwata K, Li J, Follet H, Phipps RJ, Burr DB. 2006. Bisphosphonates suppress periosteal osteoblast activity independently of resorption in rat femur and tibia. Bone. 39(5):1053–1058. [DOI] [PubMed] [Google Scholar]
- Iyer SS, Pulskens WP, Sadler JJ, Butter LM, Teske GJ, Ulland TK, Eisenbarth SC, Florquin S, Flavell RA, Leemans JC, et al. 2009. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci U S A. 106(48):20388–20393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jee JH, Lee W, Lee BD. 2010. The influence of alendronate on the healing of extraction sockets of ovariectomized rats assessed by in vivo micro-computed tomography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 110(2):e47–e53. [DOI] [PubMed] [Google Scholar]
- Kim JH, Park YB, Li Z, Shim JS, Moon HS, Jung HS, Chung MK. 2011. Effect of alendronate on healing of extraction sockets and healing around implants. Oral Dis. 17(7):705–711. [DOI] [PubMed] [Google Scholar]
- Kuiper JW, Forster C, Sun C, Peel S, Glogauer M. 2012. Zoledronate and pamidronate depress neutrophil functions and survival in mice. Br J Pharmacol. 165(2):532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroshima S, Entezami P, McCauley LK, Yamashita J. 2014. Early effects of parathyroid hormone on bisphosphonate/steroid-associated compromised osseous wound healing. Osteoporos Int. 25(3):1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroshima S, Kovacic BL, Kozloff KM, McCauley LK, Yamashita J. 2013. Intra-oral PTH administration promotes tooth extraction socket healing. J Dent Res. 92(6):553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindtner RA, Tiaden AN, Genelin K, Ebner HL, Manzl C, Klawitter M, Sitte I, von Rechenberg B, Blauth M, Richards PJ. 2014. Osteoanabolic effect of alendronate and zoledronate on bone marrow stromal cells (BMSCs) isolated from aged female osteoporotic patients and its implications for their mode of action in the treatment of age-related bone loss. Osteoporos Int. 25(3):1151–1161. [DOI] [PubMed] [Google Scholar]
- Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. 1998. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 13(4):581–589. [DOI] [PubMed] [Google Scholar]
- Miao D, Scutt A. 2002. Histochemical localization of alkaline phosphatase activity in decalcified bone and cartilage. J Histochem Cytochem. 50(3):333–340. [DOI] [PubMed] [Google Scholar]
- Pettway GJ, McCauley LK. 2008. Ossicle and vossicle implant model systems. Methods Mol Biol. 455:101–110. [DOI] [PubMed] [Google Scholar]
- Pietrokovski J, Massler M. 1971. Residual ridge remodeling after tooth extraction in monkeys. J Prosthet Dent. 26(2):119–129. [DOI] [PubMed] [Google Scholar]
- Sharma A, Pradeep AR. 2012. Clinical efficacy of 1% alendronate gel as a local drug delivery system in the treatment of chronic periodontitis: a randomized, controlled clinical trial. J Periodontol. 83(1):11–18. [DOI] [PubMed] [Google Scholar]
- Smith N. 1974. A comparative histological and radiographic study of extraction socket healing in the rat. Aust Dent J. 19(4):250–254. [DOI] [PubMed] [Google Scholar]
- Somford MP, Draijer FW, Thomassen BJ, Chavassieux PM, Boivin G, Papapoulos SE. 2009. Bilateral fractures of the femur diaphysis in a patient with rheumatoid arthritis on long-term treatment with alendronate: clues to the mechanism of increased bone fragility. J Bone Miner Res. 24(10): 1736–1740. [DOI] [PubMed] [Google Scholar]
- Toker H, Ozdemir H, Ozer H, Eren K. 2012. Alendronate enhances osseous healing in a rat calvarial defect model. Arch Oral Biol. 57(11):1545–1550. [DOI] [PubMed] [Google Scholar]
- Trombelli L, Farina R, Marzola A, Bozzi L, Liljenberg B, Lindhe J. 2008. Modeling and remodeling of human extraction sockets. J Clin Periodontol. 35(7):630–639. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Chen SM, Chen CH, Wang CK, Wang GJ, Chang JK, Ho ML. 2010. The effect of the local delivery of alendronate on human adipose-derived stem cell-based bone regeneration. Biomaterials. 31(33):8674–8683. [DOI] [PubMed] [Google Scholar]
- Yamashita J, Datta NS, Chun YH, Yang DY, Carey AA, Kreider JM, Goldstein SA, McCauley LK. 2008. Role of Bcl2 in osteoclastogenesis and PTH anabolic actions in bone. J Bone Miner Res. 23(5):621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.