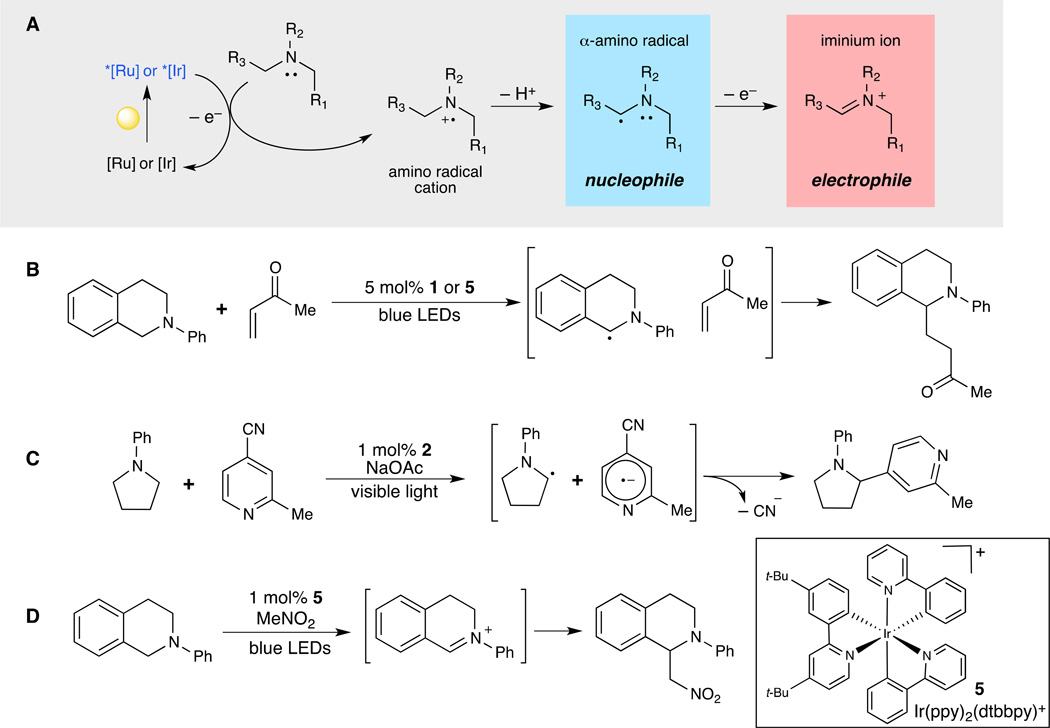
Fig. 5. Diverse reactivity of α-amino radical cations.
(A) Tertiary amines readily undergo photooxidation to yield a highly versatile amine radical cation intermediate, which can be transformed into a nucleophilic (α-amino radical) or electrophilic (iminium ion) species. (B) Pandey and Reiser were able to functionalize tetrahydroisoquinolines through the formation of an α-amino radical that readily intercepted various electrophiles (41). (C) Through the utilization of a more strongly reducing iridium photocatalyst (2) MacMillan and coworkers were able to intercept α-amino radicals with cyanoarenes, overall providing a route for α-acylating amines (43). (D) Depending upon the reaction conditions, α-amino radicals can undergo further oxidation to electrophilic iminium ions that can subsequently be trapped with nucleophilic reagents (44). Abbreviations: dtbbpy, 4,4’-di-tert-butyl-2,2’-bipyridyl; LED, light-emitting diode; Me, methyl; Ph, phenyl; ppy, 2-phenylpyridine.