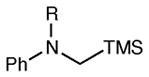
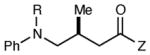
Table 2.
Reactions of Structurally Varied α-Silylamines with Michael acceptor 2b.a
| entry | amine | product | time | yield (%)b | ee (%)c |
|---|---|---|---|---|---|
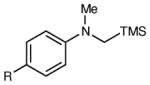
|
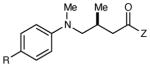
|
||||
| 1 | R = H | 6 h | 87 | 93 | |
| 2 | R = F | 6 h | 94 | 94 | |
| 3 | R = Cl | 6 h | 96 | 93 | |
| 4 | R = Br | 6 h | 90 | 92 | |
| 5 | R = Me | 12 h | 79 | 91 | |
| 6 | R = OMe | 12 h | <5 | -- | |
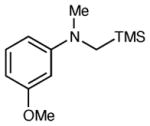
| 7 |
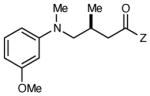
|
|
6 h | 85 | 92 |
| 8 |
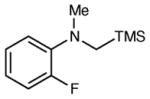
|
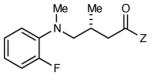
|
6 h | 80 | 96d,e |
| 9 |
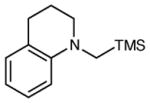
|
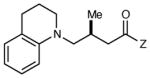
|
12 h | 80 | 90 |
| 10 |
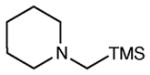
|
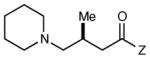
|
12 h | <5 | -- |
| 11 |
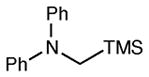
|
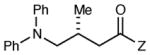
|
12 h | 93 | 91d,e |
|
|
||||
| 12 | R = Bn | 6 h | 60 | 94 | |
| 13 | R = i-Pr | 12 h | 33 | 95 | |
| 14 | R = Boc | 12 h | <5 | -- |
Unless otherwise noted, reactions were conducted using 1.5 equiv of 2, 2 mol% Ru(bpy)3Cl2, 15 mol% Sc(OTf)3, 20 mol% (S,S)-4c and 30 mol% Bu4N+Cl− in degassed MeCN (0.05 M) and were irradiated using a 23 W compact fluorescent light bulb.
Values represent the averaged isolated yields of two reproducible experiments.
Enantiomeric excess determined by chiral SFC analysis.
Enantiomeric excess of the corresponding alcohol.
Reaction conducted using (R,R)-4c to facilitate measurement of ee.
