Table 3.
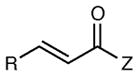
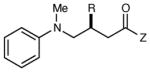
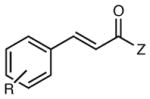
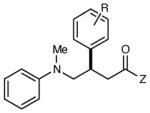
Reactions of Structurally Varied Michael Acceptors with α-Silylamine 1.a
| entry | amine | product | time | yield (%)b | ee (%)c |
|---|---|---|---|---|---|
|
|
||||
| 1 | R = n-Pr | 6 h | 76 | 93 | |
| 2 | R = i-Pr | 6 h | 71 | 93 | |
| 3 | R = CH2OBn | 6 h | 75 | 91 | |
| 4 | R = t-Bu | 12 h | 35 | 96 | |
|
|
||||
| 5 | R = H | 12 h | 74 | 93 | |
| 6 | R = p-Cl | 12 h | 63 | 91 | |
| 7 | R = p-OMe | 12 h | 83 | 94 | |
| 8 | R = o-Me | 12 h | 81 | 85 | |
| 9 |
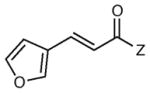
|
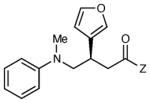
|
12 h | 83 | 91 |
Unless otherwise noted, reactions were conducted using 1.5 equiv of Michael acceptor, 2 mol% Ru(bpy)3Cl2, 15 mol% Sc(OTf)3, 20 mol% (S,S)-4c and 30 mol% Bu4N+Cl− in degassed MeCN (0.05 M) and were irradiated using a 23 W compact fluorescent light bulb.
Values represent the averaged isolated yields of two reproducible experiments.
Enantiomeric excess determined by chiral SFC analysis.
