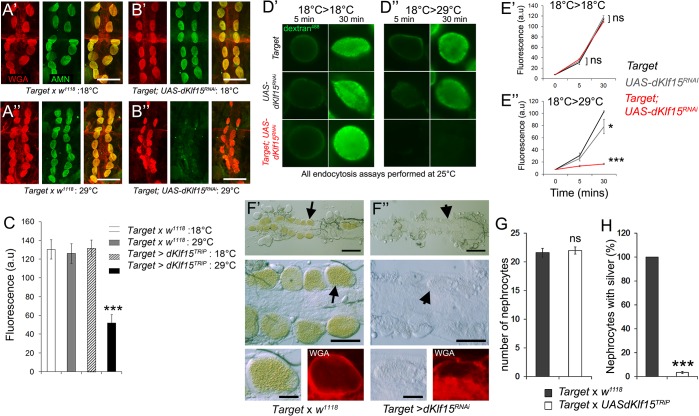
Fig 6. Adult pericardial nephrocyte function is dependent on dKlf15.
(A & B) dKlf15 was conditionally silenced in adult nephrocytes using Hand-Target (Target) to drive UAS-dKlf15 RNAi. Flies were reared at 18°C until eclosion then either maintained at 18°C (A’, B’; no knock-down) or placed at 29°C (A”, B”; RNAi permitted) for 4 days. Adult hearts were then stained with wheat germ agglutinin (red) and antibodies to the nephrocyte marker Amnionless (green). Scale bars = 100 μm. (C) Quantification of Amnionless fluorescence signal. ***P<0.001; n = 8 hearts per genotype. (D) Pericardial nephrocytes in semi-intact heart preparations after incubation with fluorescently tagged 10 kDa dextran (green) for 5 or 30 minutes. Nephrocytes of all genotypes associated with dextran when adults were kept at the non-permissive temperature of 18°C (D’), whereas at 29°C, the permissive temperature for silencing, the dKlf15 RNAi line could no longer associate with dextran (D”). (E) Quantification of fluorescence after incubation for 0, 5 or 30 minutes with fluorescently-tagged. *P<0.05, ***P<0.001compared to control (Target genotype), ns = not significantly different from control; n = 12–16 nephrocytes from 4 individual flies per genotype, per time-point. (F) Control (Target flies outcrossed to w 1118, (F’)) and Target > dKlf15 RNAi (F”) flies reared at 18°C then transferred to 29°C for 4 days and fed silver nitrate for one week. Upper and middle panels show the pericardial nephrocytes in control flies containing silver (brown pigment, arrows) but not when dKlf15 had been silenced (arrowheads). Scale bar = 100μm and 50μm. Lower panels show that pericardial nephrocytes of both genotypes could still be identified with wheat-germ agglutinin (red). Scale bar = 20μm. (G &H) Quantification of nephrocytes and the percentage of nephrocytes containing silver nitrate. ns = not significantly different from the control, ***P<0.001; n = 8 flies per genotype.