Abstract
The main objective of this study was to compare the effects of ketamine and xylazine in aging rats when coadministered intraperitoneally at high anesthetic doses. Three groups (n=6 rats/group) consisting of rats at 3, 6 and 12 months of age were used. During anesthesia, animals were monitored for heart rate, respiratory frequency, blood oxygen saturation, and rectal temperature. The corneal and paw withdrawal reflex were also examined during anesthesia. During anesthesia, withdrawal and corneal reflexes were absent for progressively longer durations with increasing age. Significant decreases in cardiac and respiratory frequency and, blood oxygen saturation occurred for the 6- and 12-month-old animals. Respiratory frequency and blood oxygen saturation returned to normal at the end of the anesthesia; however, the significant decrease in cardiac frequency persisted in the 6- and 12-month-old animals. Rectal temperature was decreased significantly only in the 3-month-old animals. Pulmonary edema and effusion occurred in 50% of the 12-month-old animals. In conclusion, if ketamine-xylazine are used for anesthesia, the doses should be optimized for the age of the subjects prior to initiation of the research project.
Keywords: aging, anesthesia, ketamine, rats, xylazine
Introduction
Ketamine and xylazine are commonly used in combination for the anesthesia of rodents [12,13,14, 25]. Ketamine is a glutamate N-Methyl-D-Aspartate (NMDA) antagonist that causes dissociative anesthesia and analgesia [40]. Opioid [30] and GABAA [37] receptors are also sites of action for ketamine, which may be associated with its sedative and analgesic properties. Xylazine is an α2-adrenergic agonist with analgesic, sedative and muscle-relaxant properties [15, 27]. Ketamine-xylazine (KX) are given together by different routes of administration, including intraperitoneally [5, 7], providing a surgical anesthesia plane and pain relief in rats. Anesthetic doses administered vary greatly in the literature with the doses being 40 to 260 mg/kg for ketamine and 5 to 39 mg/kg for xylazine when given intramuscularly, and 75 to 100 mg/kg for ketamine and 10 mg/kg for xylazine respectively when given intraperitoneally [11]. However, KX very often does not provide a surgical level of anesthesia in mice, and adding acepromazine to the mixture greatly increases its efficacy [5, 7].
The main reported negative side effects of KX are cardiac depression, bradycardia, and hypotension [7]. Both drugs cause respiratory depression, and the effects can be additive [34]. These negative side effects are mediated by central and peripheral adrenoeceptors and, muscarinic and NMDA receptors [4, 15, 28]. High doses of xylazine have been shown to cause pulmonary edema, and they may cause death in rats [1,2,3]. In a previous publication [36], we looked at the pharmacokinetics of ketamine and xylazine in young (2 months) and old (>2 years of age) Sprague Dawley rats using a high dose of ketamine (125 mg/kg) and xylazine (10 mg/kg), but we did not evaluate physiological changes during anesthesia, and few organs were collected for histopathology. In old animals, we found that the elimination half-lifes of both ketamine and xylazine were greatly increased, suggesting decreased metabolism of these drugs with aging. Liver and kidney histopathology showed no significant changes related to anesthesia, however the lungs and hearts were not subjected to histopathology. Older animals have decreased liver function, which translates into a decrease in hepatic microsomal enzyme activity that could explain the significant increase in half-lifes of the drugs. This was proposed as an explanation for prolonged xylazine-ketamine anesthesia, which may have deleterious effects as animals get older [1]. However, the progressive modifications of hepatic metabolism with aging have not been shown in relation to rodent anesthesia. A progressively shorter duration of anesthesia has been shown in rats from 1 to 3 weeks of age, with the duration of anesthesia stabilizing at 4 to 12 weeks of age. This was shown to be correlated to hepatic metabolism [38]. No KX anesthesia studies have been performed in rats of different ages ranging from 3 months to 2 years of age to see if any physiological or pathological responses occur with aging. The objective of this study was therefore to compare the depth of anesthesia and histological changes following a recommended high dose of ketamine and xylazine in Sprague Dawley rats of different ages ranging from 3 to 12 months.
Materials and Methods
Subjects
Eighteen male specific pathogen free rats Sprague Dawley (Crl:CD (SD)) originating from Charles River (St-Constant, QC, Canada) were used for this study. Two- and 3-month-old rats (n=6/age group) were purchased and kept until they were respectively 3 and 6 months old. Eight-month-old retired breeder rats were purchased and kept until they were 1 yr of age. At the time of experimentation, 3, 6 and 12 month rats weighed respectively 482 ± 17 g, 638 ± 57.5 g, and 767 ± 64.2 g. All rats were housed in a standard laboratory animal environment (fresh filtered air, 15 changes/h; temperature, 21 ± 2°C; humidity, 50 ± 20%; and light-dark cycle, 12:12 h). The rats were housed individually in ventilated cages (Green Line IVC Sealsafe Plus, Tecniplast, West Chester, PA, USA) on corn cob bedding (7097 Corncob, Harlan Teklad, Bartonville, IL, USA) changed once a week. One high-temperature polycarbonate rat retreat (Bio-serv, Flemington, NJ, USA) and one Nylabone (chew toy from Bioserv, Flemington, NJ, USA) were placed in each cage for environmental enrichment. The rats received tap water and a certified laboratory diet (2018 Teklad Global 18% Protein Rodent Diet, Harlan Teklad, Bartonville, IL, USA) ad libitum. The experimental protocol was approved by the Institutional Animal Care and Use Committee of the Ste-Justine Hospital Research Center in accordance with the guidelines of the Canadian Council on Animal Care [8].
Treatments
All rats received intraperitoneally 125 mg/kg of ketamine (Ketalean, Bimeda-MTC, Cambridge, ON, Canada) and 10 mg/kg of xylazine (Xylamax, Bimeda-MTC, Cambridge, ON, Canada). These doses were selected from a previous study on the effects of ketamine and xylazine in aging animals [36].
Evaluation of anesthesia depth
Following the KX injection, different parameters were evaluated at selected time points (5, 15, 30 and 45 min, 1 h, and every half hour thereafter) until a paw withdrawal reflex was observed. Rectal temperature was monitored (Thermalert TH-8, Physitemp, Clifton, NJ, USA) and a small animal oximeter (CANL-425V, Med Associates, St. Alban, VT, USA) was used for monitoring cardiac frequency and blood oxygen saturation by taping the sensor to the right hind paw. Respiratory frequency was monitored by direct observation (over 1 min). The corneal reflex (always evaluated by MCG) was evaluated by gently pressing on the cornea with a cotton tip covered with a non-medicated ophthalmic gel.
The anesthesia duration was evaluated as the period from loss to recovery of the paw withdrawal reflex (always evaluated by MCG), which was evaluated by pressing interdigital hind paw skin with hemostatic forceps in a manner that would not cause tissue damage. The recovery time was measured as the time until the first voluntary movement following the KX injection.
Statistical analysis
An analysis of variance (ANOVA) linear model with repeated measures was performed with SAS (version 9.3, SAS Institute Inc., Cary, NC, USA) to evaluate the effect of time (within group variance) for rectal temperature, heart rate, respiratory frequency, and oxygen saturation. To compare the age effect, an ANOVA linear model with repeated measures was used, and a priori contrasts were done to compare the means of each group with an adjustment of alpha with a Bonferroni sequential correction. Groups were compared for 15, 30, and 45 min results, since all animals showed no withdrawal reflex at these time points.
The statistical significance level was set a priori at P<0.05. Data in the article and figures are reported as means (± SE).
Results
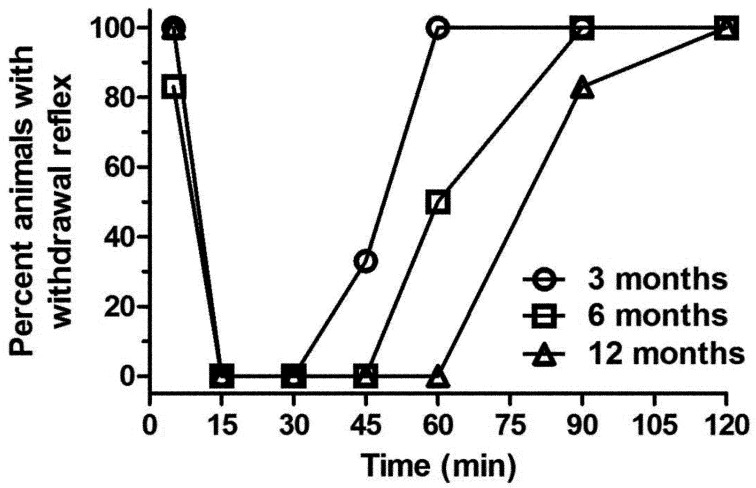
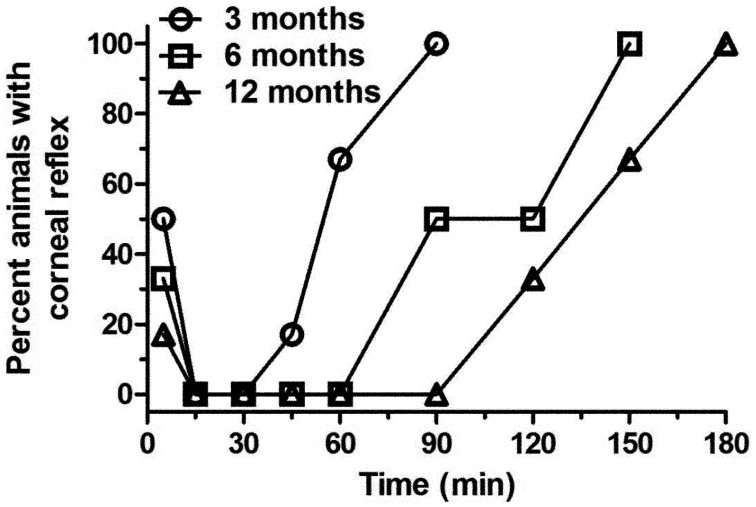
The withdrawal reflex (WR) results (Fig. 1) show a clear difference in anesthesia duration between different age groups following the administration of KX. The WR was absent in the 3-, 6-, and 12-month-old animals for 15, 30, and 45 min respectively, and all animals recovered at 60, 90, and 120 min respectively. The most apparent changes were seen at 60 min with all the 3-month-old rats recuperating by that time and the WR absent in the 12-month-old rats. The corneal reflex (Fig. 2) followed the same pattern: the reflex was absent from 15 to 60, 15 to 90 and 15 to 120 min and fully recovered at 90, 150, and 180 min in the 3-, 6-, and 12-month-old rats respectively. The most apparent changes were seen at 60 min, where there is a clear difference between the 3 month old rats and older animals, and at 90 min with a clear difference between the 3- and 12-month-old rats. First voluntary movements were noted at 85.6 ± 23.3, 123.4 ± 40.0, and 150.5 ± 24.5 min for the 3-, 6-, and 12-month-old rats respectively. The results for the 6- and 12-month-old rats were significantly different from those of the 3 month-old-rats (P<0.05 and P<0.001, respectively).
Fig. 1.
Percent of Sprague Dawley rats (n=6/group: 3, 6, and 12 months old) showing a positive withdrawal reflex when evaluated at selected time points (5, 15, 30, and 45 min, and 1, 1.5, and 2 h) following intraperitoneal administration of 125 mg/kg of ketamine and 10 mg/kg xylazine.
Fig. 2.
Percent of Sprague Dawley rats (n=6/group: 3, 6, and 12 months old) showing a positive corneal reflex when evaluated at selected time points (5, 15, 30, and 45 min, and 1, 1.5, and 2 h) following intraperitoneal administration of 125 mg/kg of ketamine and 10 mg/kg xylazine.
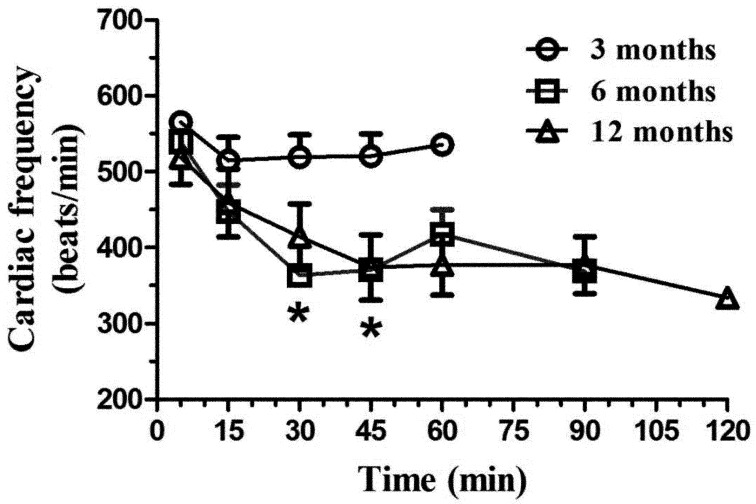
Heart rate did not vary with time for the 3-month-old animals (F4,20=2.35, P=0.09), but did vary for the 6- (F5,25=10.57, P<0.0001) and 12-month-old rats (F6,29=7.13, P<0.0001). The 6- and 12-month-old rats had a significantly decreased heart rate (Fig. 3) that did not return to normal at the end of anesthesia. Significant age group differences were found between the 3-month-old group and older animals (P<0.01 at 30 and 45 min).
Fig. 3.
Mean (± SE) heart rate (beats/min) in Sprague Dawley rats (n=6/age group: 3, 6, and 12 months old) following intraperitoneal administration of 125 mg/kg of ketamine and 10 mg/kg xylazine. The heart rate was evaluated with a pulse oximeter at selected time points (5, 15, 30, and 45 min, and 1, 1.5, and 2 h), with the evaluations ending when animals had a positive withdrawal reflex. *P<0.01.
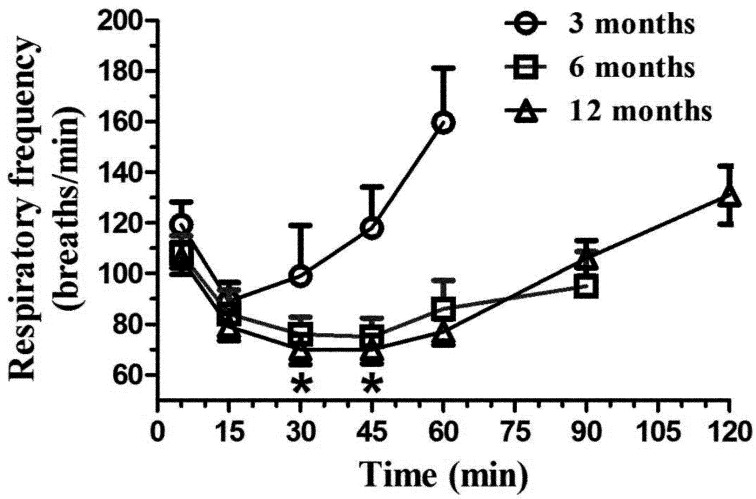
Respiratory frequency (Fig. 4) varied with time for the 3- (F4,20=5.33, P<0.005), 6-, (F5,25=3.29, P<0.03) and 12-month-old rats (F6,29=16.2, P<0.0001). All groups had a significantly decreased respiratory frequency at 15 min however this decrease persisted only for 6- and 12-month-old rats at 15 and 30 post KX as well as at 60 min for the 12-month-old animals. Significant age group differences occurred between the 3-month-old animals and other animals (P<0.05 at 30 and 45 min).
Fig. 4.
Mean (± SE) respiratory frequency (breaths/min) in Sprague Dawley rats (n=6/age group: 3, 6, and 12 months old) following intraperitoneal administration of 125 mg/kg of ketamine and 10 mg/kg xylazine. The respiratory frequency was evaluated at selected time points (5, 15, 30, and 45 min, and 1, 1.5, and 2 h), with the evaluations ending when animals had a positive withdrawal reflex. *P<0.05.
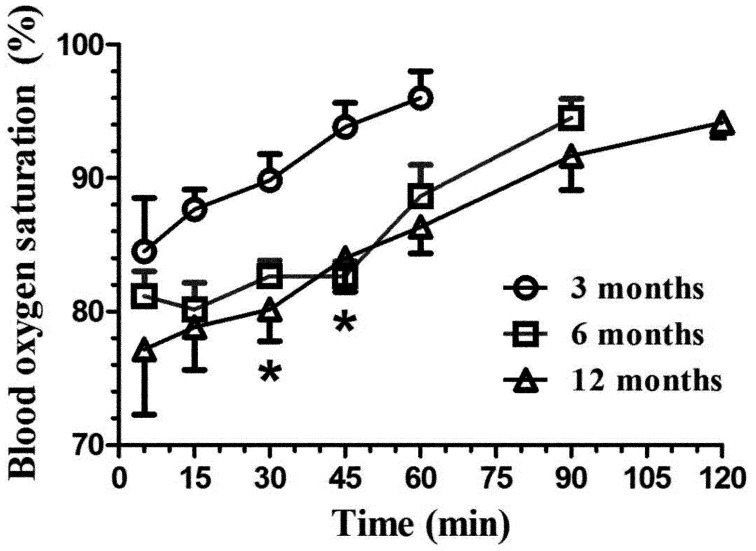
Blood oxygen saturation (Fig. 5) varied with time for the 3- (F4,20=3.07, P<0.05), 6-, (F5,25=9.78, P<0.0001) and 12-month-old rats (F6,29=5.13, P<0.002). All groups had significant blood oxygen saturation decreases; however, the 6- and 12-month-old animals were the most affected. Interestingly, all groups returned to near normal oxygen saturation levels upon awakening (94–96%). Significant age group differences occurred between the 3-month-old group and other animals at 30 and 45 min (P<0.02).
Fig. 5.
Mean (± SE) blood oxygen saturation (%) in Sprague Dawley rats (n=6/age group: 3, 6, and 12 months old) following intraperitoneal administration of 125 mg/kg of ketamine and 10 mg/kg xylazine. The SaO2 was evaluated with a pulse oximeter at selected time points (5, 15, 30, and 45 min, and 1, 1.5, and 2 h), with the evaluations ending when animals had a positive withdrawal reflex. *P<0.02.
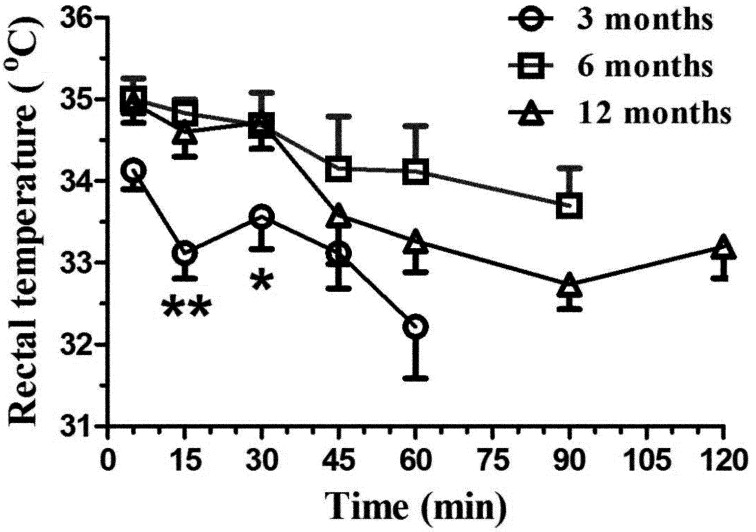
Rectal temperature (Fig. 6) varied with time for the 3- (F4,20=3.11, P<0.04) and 12-month-old rats (F6,29=5.57, P<0.001) but not for the 6-month-old rats (F5,25=1.40, P=0.26). Rectal temperature was most significantly decreased in the 12-month-old animals. No effect was observed in the 6-month-old animals; however a significant decrease was seen for the 3-month-old animals at 60 min post KX. Significant age group differences were found between the 3-month-old group and other animals at 15 and 30 min (P<0.001 and 0.02 respectively).
Fig. 6.
Mean (± SE) rectal temperature (°C) in Sprague Dawley rats (n=6/age group: 3, 6, and 12 months old) following intraperitoneal administration of 125 mg/kg of ketamine and 10 mg/kg xylazine. The rectal temperature was evaluated with a pulse oximeter at selected time points (5, 15, 30, and 45 min, and 1, 1.5, and 2 h), with the evaluations ending when animals had a positive withdrawal reflex. ** P<0.001, * P<0.02.
The three-month-old rats recovered normally following anesthesia and appeared normal the next day. Although all 6-month-old rats appeared to recover normally following the anesthesia, one had to be euthanized at 9 h following the end of the anesthesia because it progressively deteriorated (no spontaneous movement, shallow breathing). The same observations were noted for the 12-month-old rats except that 3 animals had to be euthanized at 6 h following the end of the anesthesia. The remaining 6- and 12-month-old animals were considered normal at 24 h following anesthesia.
Figure 7 shows the macroscopic appearance of typical findings of the thoracic cage of 12-month-old affected rats. There was a moderate to significant amount of bilateral serous pleural effusion (hydrothorax). The heart appeared enlarged with a globular shape. The lungs had a diffusely glistening pleural surface (consistent with edema), with some patchy gray-red discoloration. No effusion or cardiomegaly was observed in 3- and 6-month-old rats.
Fig. 7.
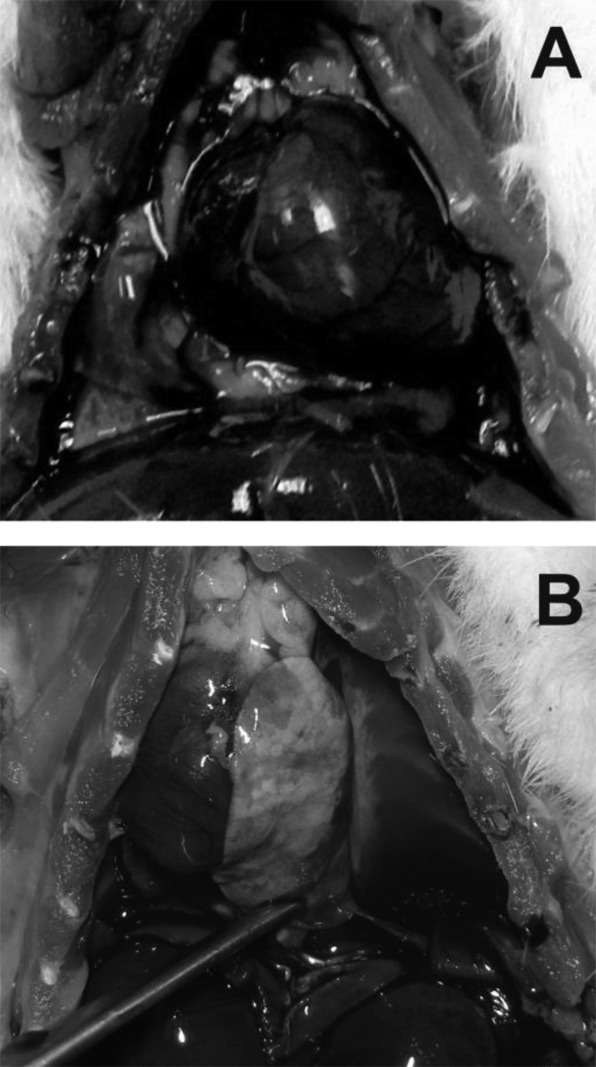
Photographs of the thoracic cavity of two 12-month-old rats. A. The heart appears enlarged with a globular shape. A & B. The lungs have a diffusely glistening pleural surface, with some patchy gray-red discoloration. A moderate to significant amount of serous pleural effusion can be seen. B. The lung is pushed to the side (with tweezers) to appreciate the amount of pleural effusion.
The microscopic findings for lung tissue (Fig. 8) included alveolar and perivascular edemas in the 6- and 12-month-old affected rats. This was also seen in one in the 3-months-old rats, but the edema was localized to the hilum of the lung only. No or incidental histopathological findings were observed in other organs. Mild myocardial fibrosis and necrosis were observed in one 3-month-old rat and two rats of each of the other groups. The liver and kidneys were normal.
Fig. 8.
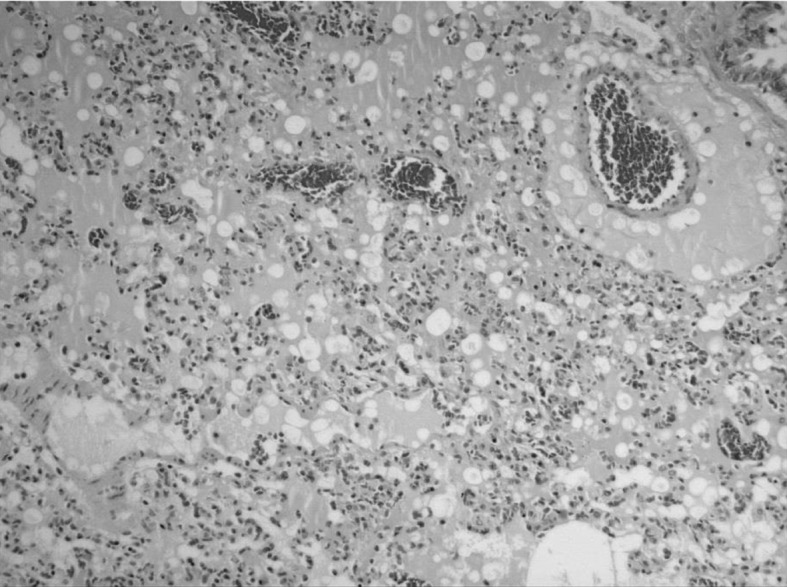
Photomicrograph showing perivascular and alveolar edema seen in the 12-month-old animals (×100).
Discussion
In this study, although the duration of anesthesia increased with age, within reasonable time limits (15 to 45 min), the time to recovery was very long in aged animals when considering the withdrawal and corneal reflexes as well as the time to the first voluntary movement. This was especially true for the 6- and 12-month-old rats. These animals showed a decreased cardiac frequency that did not return to baseline at the end of the observation period. Although the respiratory frequency and blood oxygen saturation were also depressed significantly during anesthesia, the 6- and 12-month-old animals recuperated to near normal baseline values at the end of the observation period. More importantly, one animal in the 6-month-old group, and 3 animals in the 12-month-old group, needed to be euthanized due to poor recovery form anesthesia as well as lung edema and pulmonary effusion. Therefore, these results suggest that much lower anesthetic doses need to be administered with increasing age to attain a safe level of anesthesia. In our previous study [36], we did not observe these deleterious pulmonary effects following high doses of KX in old animals (>2 yrs); however, the animals in the previous study were healthy aged rats (many others in the aging colony had already died of noninfectious causes), and this could possibly reflect a selection bias. Importantly, not all 18-month-old rats were equally affected (only 50%), something we did not observe in the first study [36], which explains why we did not perform cardiac and pulmonary histopathology in these animals.
The most frequently used injectable anesthetic combination in rodents is ketamine and xylazine [6, 32]. Ketamine has been associated with many advantages including a simple route of administration, greater margin of safety, and the possibility of being combined with other drugs [13]. Our data suggests that the margin of safety needs to be revised in light of these adverse effects. Xylazine is mainly used as a sedative and an analgesic drug [18, 19]. High doses of xylazine (42 mg/kg) in young animals cause pulmonary edema [1,2,3], and this toxicity is independent of ketamine [2]. We previously showed [36] that xylazine clearance was greatly decreased in old animals, suggesting that it could attain toxic levels since greater drug exposure occurs. Both ketamine and xylazine are metabolized by the liver cytochromes P450 enzymes [16, 21] and changes in liver metabolism with aging could very well explain our findings [39]. Ultrastructural and biochemical changes in hepatocytes occur with aging [29] in association with changes in cytochrome P450 enzymes [26]. Future experiments in our laboratory will address the importance of hepatic metabolism of anesthetic drugs in aging rats.
Xylazine is extensively metabolized into many metabolites [23]; however, up to 70% of xylazine is eliminated in urine [24]. Ketamine is mainly transformed into an active metabolite (norketamine), and these molecules are also eliminated in urine [21]. Increased plasmatic concentrations with aging could also be due to a decrease in glomerular filtration rates, although the total number of nephrons does not seem to be altered with aging [9, 10]. Although kidney lesions are associated with aging in Sprague Dawley rats, we did not see any anomalies even in our 12-month-old age group, and this suggests that kidney malfunction is probably not associated with microscopic changes; however, drug metabolism also occurs in the kidneys, and this could be modified with aging.
Many factors can affect the distribution of drugs such as age, sex, nutrition, environmental conditions, and diseases [17, 20, 31, 33, 35]. Aging could also affect drug metabolism due to chronic subclinical inflammation, obesity and diminished exercise [22]. Both ketamine and xylazine are lipophilic drugs that are stored in fat tissues, and this obviously alters the pharmacokinetics as fat deposits increase with age. These factors will need to be integrated into future drug metabolism studies.
In conclusion, age and associated factors have a significant effect on ketamine and xylazine anesthesia. High doses of ketamine and xylazine increased the duration of anesthesia; however, deleterious effects were mainly seen in the heart function (cardiac depression) and the lungs (pulmonary edema and effusion) of 6- and 12- month-old rats. These high doses should not be recommended for anesthesia in these age groups, and if these drugs need to be used for a specific research protocol, optimization of the proper doses is strongly recommended before the study is performed. The choice of anesthetic regimen may vary with different models according to the target organ (i.e., liver or kidney) or the disease process (i.e., inflammation or cancer). Our recent findings (unpublished data) shows that if KX is used in aging animals, with uncomplicated disease processes, a dose of 80 mg/kg of ketamine and 10 mg/kg of xylazine will not affect certain organs (liver, kidney, heart, and lungs). We would therefore recommend these doses in aged Sprague Dawley rats.
Acknowledgments
The authors would like to thank Dr. Guy Beauchamp, a statistician in the Faculty of Veterinary Medicine, for the statistical analyses. This study was supported by the Fond de Développement pour la Médecine des Animaux de Laboratoire (Pascal Vachon) and the Fond du Centenaire de Faculté de Médecine Vétérinaire.
References
- 1.Amouzadeh H.R., Sangiah S., Qualls C.W., Jr. 1989. Effects of some hepatic microsomal enzyme inducers and inhibitors on xylazine-ketamine anesthesia. Vet. Hum. Toxicol. 31: 532–534. [PubMed] [Google Scholar]
- 2.Amouzadeh H.R., Sangiah S., Qualls C.W., Jr, Cowell R.L., Mauromoustakos A.1991. Xylazine-induced pulmonary edema in rats. Toxicol. Appl. Pharmacol. 108: 417–427. doi: 10.1016/0041-008X(91)90088-V [DOI] [PubMed] [Google Scholar]
- 3.Amouzadeh H.R., Qualls C.W., Jr, Wyckoff J.H., 3rd, Dzata G.K., Sangiah S., Mauromoustakos A., Stein L.E.1993. Biochemical and morphological alterations in xylazine-induced pulmonary edema. Toxicol. Pathol. 21: 562–571. doi: 10.1177/019262339302100607 [DOI] [PubMed] [Google Scholar]
- 4.Antonaccio M.J., Robson R.D., Kerwin L.1973. Evidence for increased vagal tone and enhancement of baroreceptor reflex activity after xylazine (2-(2,6-dimethylphenylamino)-4-H-5,6-dihydro-1,3-thiazine) in anesthestized dogs. Eur. J. Pharmacol. 23: 311–316. doi: 10.1016/0014-2999(73)90102-7 [DOI] [PubMed] [Google Scholar]
- 5.Arras M., Autenried P., Rettich A., Spaeni D., Rülicke T.2001. Optimization of intraperitoneal injection anesthesia in mice: drugs, dosages, adverse effects, and anesthesia depth. Comp. Med. 51: 443–456. [PubMed] [Google Scholar]
- 6.Branson K.R. 2001. Injectables anesthetics, p.213–267, In: Adams HR, editor. Veterinary Pharmacology and Therapeutics. Ames, Iowa State Press. [Google Scholar]
- 7.Buitrago S., Martin T.E., Tetens-Woodring J., Belicha-Villanueva A., Wilding G.E.2008. Safety and efficacy of various combinations of injectable anesthetics in BALB/c mice. J. Am. Assoc. Lab. Anim. Sci. 47: 11–17. [PMC free article] [PubMed] [Google Scholar]
- 8.Canadian Council on Animal Care1993. Guide to the care and use of experimental animals, vol 1, 2nd ed. Ottawa (Canada): Canadian Council on Animal Care. [Google Scholar]
- 9.Corman B., Pratz J., Poujeol P.1985. Changes in anatomy, glomerular filtration, and solute excretion in aging rat kidney. Am. J. Physiol. 248: R282–R287. [DOI] [PubMed] [Google Scholar]
- 10.Corman B., Michel J.B.1987. Glomerular filtration, renal blood flow, and solute excretion in conscious aging rats. Am. J. Physiol. 253: R555–R560. [DOI] [PubMed] [Google Scholar]
- 11.Dittmar M.S., Fehm N.P., Vatankhah B., Horn M.2004. Ketamine/xylazine anesthesia for radiologic imaging of neurologically impaired rats: dose response, respiratory depression, and management of complications. Comp. Med. 54: 652–655. [PubMed] [Google Scholar]
- 12.Flecknell P.A.1996. Laboratory animal anesthesia. Academic Press, London. [Google Scholar]
- 13.Gaertner D.J., Hallman T.M., Hankenson F.C., Batchelder M.A. 2008. Anesthesia and Analgesia for Laboratory Rodents, p.240–282, In: Fish RE, Brown MJ, Danneman PJ, Karas AZ, editors. Anesthesia and Analgesia in Laboratory Animals. San Diego, Academic Press. [Google Scholar]
- 14.Green C.J., Knight J., Precious S., Simpkin S.1981. Ketamine alone and combined with diazepam or xylazine in laboratory animals: a 10 year experience. Lab. Anim. 15: 163–170. doi: 10.1258/002367781780959107 [DOI] [PubMed] [Google Scholar]
- 15.Greene S.A., Thurmon J.C.1988. Xylazine—a review of its pharmacology and use in veterinary medicine. J. Vet. Pharmacol. Ther. 11: 295–313. doi: 10.1111/j.1365-2885.1988.tb00189.x [DOI] [PubMed] [Google Scholar]
- 16.Hijazi Y., Boulieu R.2002. Contribution of CYP3A4, CYP2B6, and CYP2C9 isoforms to N-demethylation of ketamine in human liver microsomes. Drug Metab. Dispos. 30: 853–858. doi: 10.1124/dmd.30.7.853 [DOI] [PubMed] [Google Scholar]
- 17.Jenkins A.J. 2007. Toxicokinetics and Factors Affecting Pharmacokinetic Parameters, p. 21–24, In: Karch SB, editor. Pharmacokinetics and Pharmacodynamics of Abused Drugs. CRC Press. [Google Scholar]
- 18.Kästner S.B.2006. A2-agonists in sheep: a review. Vet. Anaesth. Analg. 33: 79–96. doi: 10.1111/j.1467-2995.2005.00243.x [DOI] [PubMed] [Google Scholar]
- 19.Lamont L.A., Tranquilli W.J., Grimm K.A.2000. Physiology of pain. Vet. Clin. North Am. Small Anim. Pract. 30: 703–728, v. doi: 10.1016/S0195-5616(08)70003-2 [DOI] [PubMed] [Google Scholar]
- 20.Majewski-Tiedeken C.R., Rabin C.R., Siegel S.J.2008. Ketamine exposure in adult mice leads to increased cell death in C3H, DBA2 and FVB inbred mouse strains. Drug Alcohol Depend. 92: 217–227. doi: 10.1016/j.drugalcdep.2007.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer R.E., Fish R.E. 2008. Pharmacology of injectable anesthetics, sedatives, and tranquilizers, p. 27–82, In: Fish RE, Brown MJ, Danneman PJ, Karas AZ, editors. Anesthesia and Analgesia in Laboratory Animals. San Diego, Academic Press. [Google Scholar]
- 22.Mézière A., Paillaud E., Plaud B.2013. Anesthésie de la personne âgée. (in French). Presse Med. 42: 197–201. [DOI] [PubMed] [Google Scholar]
- 23.Mössner L.D., Schmitz A., Theurillat R., Thormann W., Mevissen M.2011. Inhibition of cytochrome P450 enzymes involved in ketamine metabolism by use of liver microsomes and specific cytochrome P450 enzymes from horses, dogs, and humans. Am. J. Vet. Res. 72: 1505–1513. doi: 10.2460/ajvr.72.11.1505 [DOI] [PubMed] [Google Scholar]
- 24.Park Choo H.Y., Choi S.O.1991. The metabolism of xylazine in rats. Arch. Pharm. Res. 14: 346–351. doi: 10.1007/BF02876882 [DOI] [Google Scholar]
- 25.Richardson C.A., Flecknell P.A.2005. Anaesthesia and post-operative analgesia following experimental surgery in laboratory rodents: are we making progress? Altern. Lab. Anim. 33: 119–127. [DOI] [PubMed] [Google Scholar]
- 26.Rikans L.E., Notley B.A.1982. Age-related changes in hepatic microsomal drug metabolism are substrate selective. J. Pharmacol. Exp. Ther. 220: 574–578. [PubMed] [Google Scholar]
- 27.Salonen J.S.1992. Chemistry and pharmacokinetics of the alpha2-adrenoreceptor agonist, p.191–200, In: Short CE, Van Poznak A, editors. Animal Pain. New York, Churchill Livingstone. [Google Scholar]
- 28.Schmitt H., Fournadjiev G., Schmitt H.1970. Central and peripheral effects of 2-(2,6-dimethylphenylamino)-4-H-5,6-dihydro-1,3-thiazin (Bayer 1470) on the sympathetic system. Eur. J. Pharmacol. 10: 230–238. doi: 10.1016/0014-2999(70)90277-3 [DOI] [PubMed] [Google Scholar]
- 29.Schmucker D.L.1990. Hepatocyte fine structure during maturation and senescence. J. Electron Microsc. Tech. 14: 106–125. doi: 10.1002/jemt.1060140205 [DOI] [PubMed] [Google Scholar]
- 30.Smith D.J., Pekoe G.M., Martin L.L., Coalgate B.1980. The interaction of ketamine with the opiate receptor. Life Sci. 26: 789–795. doi: 10.1016/0024-3205(80)90285-4 [DOI] [PubMed] [Google Scholar]
- 31.Song G., Wu H., Yoshino K., Zamboni W.C.2012. Factors affecting the pharmacokinetics and pharmacodynamics of liposomal drugs. J. Liposome Res. 22: 177–192. doi: 10.3109/08982104.2012.655285 [DOI] [PubMed] [Google Scholar]
- 32.Stokes E.L., Flecknell P.A., Richardson C.A.2009. Reported analgesic and anaesthetic administration to rodents undergoing experimental surgical procedures. Lab. Anim. 43: 149–154. doi: 10.1258/la.2008.008020 [DOI] [PubMed] [Google Scholar]
- 33.Struck M.B., Andrutis K.A., Ramirez H.E., Battles A.H.2011. Effect of a short-term fast on ketamine-xylazine anesthesia in rats. J. Am. Assoc. Lab. Anim. Sci. 50: 344–348. [PMC free article] [PubMed] [Google Scholar]
- 34.Schwenke D.O., Cragg P.A.2004. Comparison of the depressive effects of four anesthetic regimens on ventilatory and cardiovascular variables in the guinea pig. Comp. Med. 54: 77–85. [PubMed] [Google Scholar]
- 35.Veilleux-Lemieux D., Beaudry F., Hélie P., Vachon P.2012. Effects of endotoxemia on the pharmacodynamics and pharmacokinetics of ketamine and xylazine anesthesia in Sprague-Dawley rats. Vet. Med. Res. Rep. 3: 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Veilleux-Lemieux D., Castel A., Carrier D., Beaudry F., Vachon P.2013. Pharmacokinetics of ketamine and xylazine in young and old Sprague-Dawley rats. J. Am. Assoc. Lab. Anim. Sci. 52: 567–570. [PMC free article] [PubMed] [Google Scholar]
- 37.Velísková J., Velísek L., Mares P., Rokyta R.1990. Ketamine suppresses both bicuculline- and picrotoxin-induced generalized tonic-clonic seizures during ontogenesis. Pharmacol. Biochem. Behav. 37: 667–674. doi: 10.1016/0091-3057(90)90544-R [DOI] [PubMed] [Google Scholar]
- 38.Waterman A.E., Livingston A.1978. Effects of age and sex on ketamine anaesthesia in the rat. Br. J. Anaesth. 50: 885–889. doi: 10.1093/bja/50.9.885 [DOI] [PubMed] [Google Scholar]
- 39.Wauthier V., Verbeeck R.K., Buc Calderon P.2004. Age-related changes in the protein and mRNA levels of CYP2E1 and CYP3A isoforms as well as in their hepatic activities in Wistar rats. What role for oxidative stress? Arch. Toxicol. 78: 131–138. doi: 10.1007/s00204-003-0526-z [DOI] [PubMed] [Google Scholar]
- 40.White P.F., Way W.L., Trevor A.J.1982. Ketamine—its pharmacology and therapeutic uses. Anesthesiology 56: 119–136. doi: 10.1097/00000542-198202000-00007 [DOI] [PubMed] [Google Scholar]