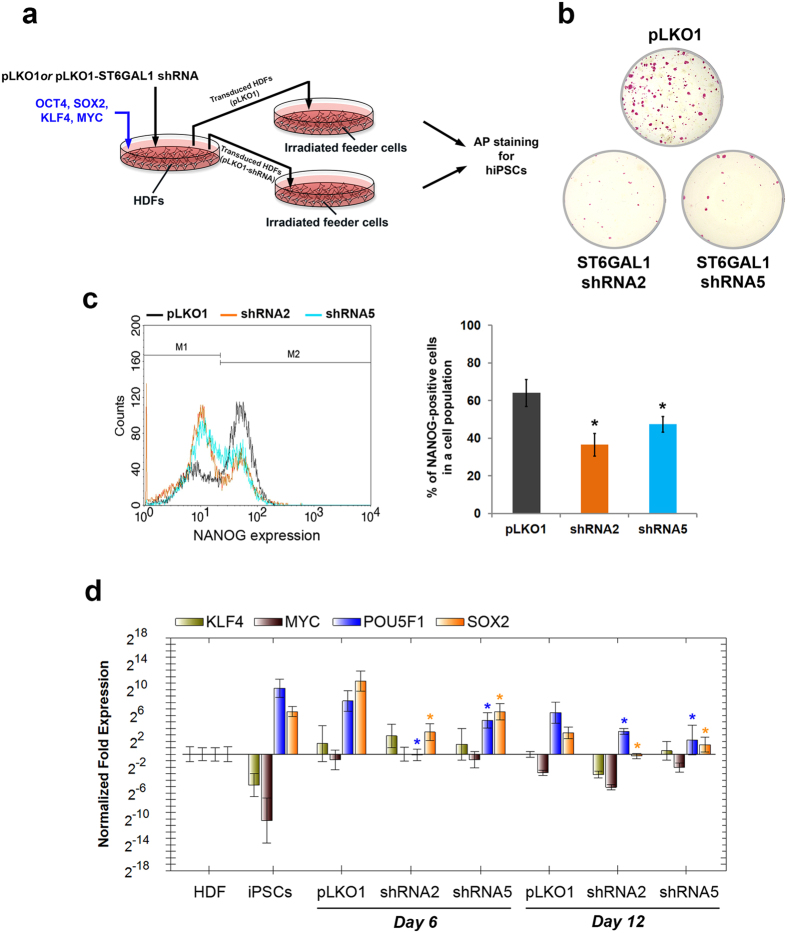
Figure 3. ST6GAL1 knockdown impedes cellular reprogramming and establishment of induced pluripotency in human somatic cells.
(a) Schematic illustration of the experimental strategy to examine the influence of ST6GAL1 knockdown on cellular reprogramming. Twenty-four hours after HDFs received POU5F1, SOX2, KLF4, and MYC with or without ST6GAL1 shRNA, the transduced HDFs were seeded on X-ray irradiated feeder cells (mouse embryonic fibroblasts, MEFs). Fourteen days later, alkaline phosphatase (AP) staining was used to examine hiPSC colonies formed by transduced HDFs on the feeder cells. (b) AP staining showed that dramatically fewer hiPSC colonies with AP activity were obtained from cellular reprogramming under ST6GAL1 knockdown mediated by shRNA2 and shRNA5. (c) Quantitative analysis of NANOG expressing cells in the reprogrammed cell population showed that shRNA2 and shRNA5 both led to a significant reduction in NANOG expressing cells in the analyzed cell populations. Left Panel: The histogram representation of flow cytometry analysis. Right panel: the quantitative result of flow cytometry analysis (n = 3; *P < 0.05, t-test). (d) The expression of endogenous POU5F1, SOX2, KLF4 and MYC genes in cells that underwent reprogramming with or without ST6GAL1 knockdown was measured by qRT-PCR with primer sets that target untranslated regions of the endogenous gene transcripts. The induction of endogenous POU5F1 and SOX2 gene expression at the early stage of cellular reprogramming in HDFs was substantially suppressed by shRNA-mediated ST6GAL1 knockdown (n = 3; *P < 0.05, t-test). Day 6: cell samples collected at 6 days after the initial transduction (2 days after the beginning of puromycin selection). Day 12: cell samples collected at 12 days after the initial transduction (8 days after the beginning of puromycin selection).