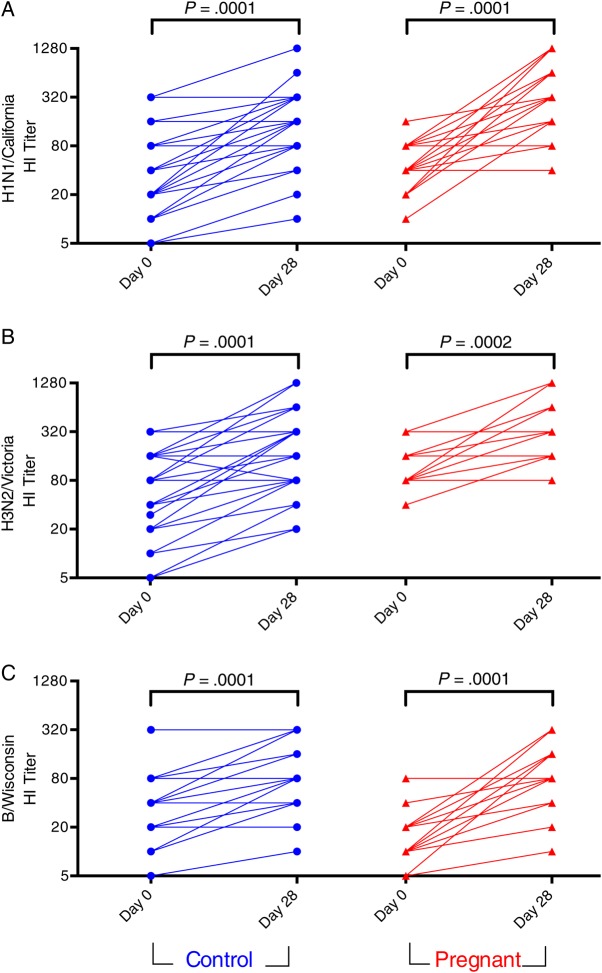
Figure 1.
HI titers to A/H1N1/California/2009 (pH1N1) (A), A/H3N2/Victoria/2011 (B), and B/Wisconsin/2010 (C) before (Day 0) and after (Day 28) IIV administration in pregnant and control women. Titers are the reciprocal of the highest serum dilution capable of preventing hemagglutination of red blood cells. Lines connect data points from individuals. Data points represent average of technical replicates. Abbreviations: HI, hemagglutination inhibition; IIV, inactivated influenza vaccine.