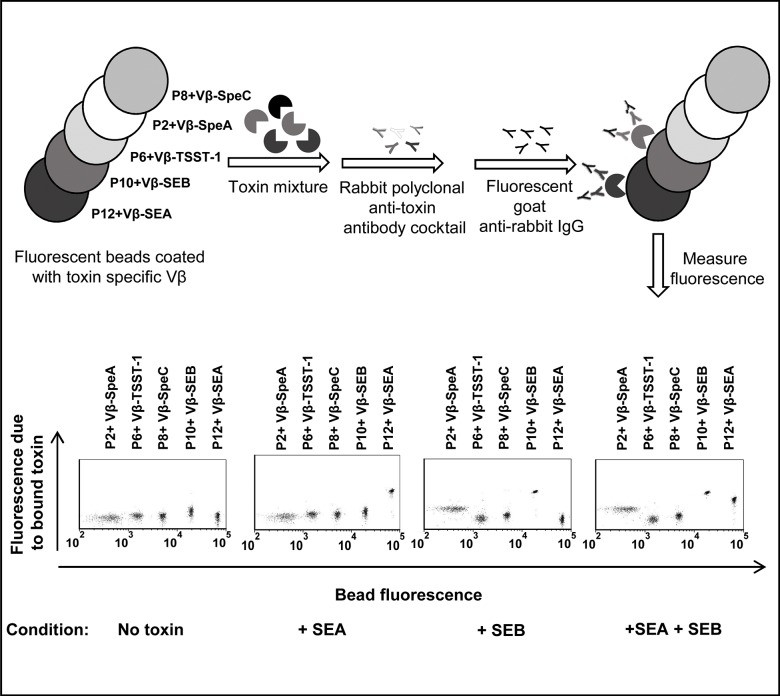
Fig 1. Schematic diagram and representative flow cytometry data of the multiplex assay.
Biotinylated, high-affinity Vβ proteins (Vβ-SEA, Vβ-SEB, Vβ-TSST-1, Vβ-SpeA and Vβ-SpeC) were immobilized on streptavidin-coated fluorescent beads (P12, P10, P6, P2 and P8 respectively, each having unique fluorescence in the FL-1 channel, λemission = 525 nm). For detecting the presence of one or more toxins (SEA and/or SEB in this example), a mixture of Vβ immobilized-beads was added to an unknown sample. Toxins captured by the Vβ-immobilized beads were detected by rabbit polyclonal anti-toxin antibodies followed by goat-anti rabbit IgG labeled with Alexa fluor 647 (λemission = 688 nm in FL-4 channel). Scatter plots in the absence or presence of toxin(s) are shown in the lower panel. An increase in fluorescence emitted by the beads in the FL-4 channel (Y-axis) indicates the presence of toxin(s) in the unknown sample. Since each class of beads emits distinct fluorescence in FL-1 channel (X-axis), identity of the toxin present is established by identifying the specific, Vβ-immobilized bead(s) undergoing an increase in fluorescence in the FL-4 channel (Y-axis). In the presence of SEA, only the P12 beads (immobilized with Vβ-SEA) exhibited an increase in fluorescence along Y-axis. In the presence of SEB, P10 beads (immobilized with Vβ-SEB) as well as P2 beads (immobilized with Vβ-SpeA) exhibited increases in fluorescence along Y-axis, due to low affinity of Vβ-SpeA toward SEB. Accordingly, in the presence of SEA and SEB, Vβ-immobilized P12, P10 and P2 beads exhibited increases in fluorescence along Y-axis.