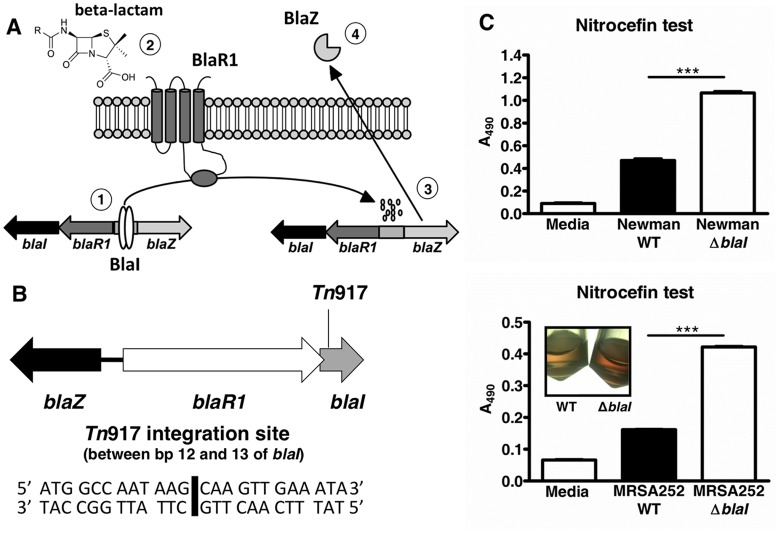
Fig 1. Mapping of the Tn917 mutant and subsequent inactivation of blaI leading to elevated beta-lactamase production.
(A) (1) In the absence of beta-lactams, the blaZ-blaR1-blaI genes are repressed by BlaI. (2) When beta-lactam molecules are sensed by BlaR1, the cytoplasmatic domain of this transmembrane protein is autoproteolytically cleaved. (3) Following this event, the repressor protein BlaI is proteolytically cleaved and dissociates from its binding site, enabling transcription of the beta-lactamase-encoding gene blaZ. (4) Finally, the active beta-lactamase BlaZ is secreted, leading to hydrolysis of the beta-lactam molecules. (B) Organization of the blaZ-blaR1-blaI locus in S. aureus Newman. blaR1 and blaI are located in a two-gene operon. blaZ is divergently transcribed. In the cathelicidin susceptible S. aureus Newman mutant G2E3, Tn917 integration occurred 12 bp downstream of the blaI start codon. (C) S. aureus Newman WT and blaI mutant or MRSA252 WT and blaI mutant were incubated with 50 μg/ml nitrocefin for 30 min at 37°C, and the absorbance at 490 nm (A490) was read. A higher A490 value reflects higher beta-lactamase activity. Mean A490 values ± SD of duplicates of one representative experiment of at least three performed for each strain are shown. ***, p<0.001. A representative picture for the MRSA252 WT and blaI mutant bacteria after incubation with nitrocefin are shown.