Abstract
Liver tolerance was initially recognized by the spontaneous acceptance of liver allograft in many species. The underlying mechanisms are not completely understood. We have been inspired by an unexpected phenomenon that the liver transplant tolerance absolutely requires interferon (IFN)-γ, a rejection-associated inflammatory cytokine. In this study, we investigate the rejection of liver allografts deficient in IFN-γ receptor and reveal that the liver graft is equipped with machineries capable of counterattacking the host immune response through a mesenchyme-mediated immune control (MMIC) mechanism. MMIC is triggered by T effectors (Tef) cell-derived IFN-γ to drive the expression of B7-H1 on graft mesenchymal cells leading to Tef cell apoptosis. We describe the negative feedback loop between graft mesenchymal and Tef cells that ultimately results in liver transplant tolerance. Comparable elevations of T regulatory cells and myeloid-derived suppressor cells are seen in both rejection and tolerance groups, and are not dependent on IFN-γ stimulation, suggesting a critical role of Tef cell elimination in tolerance induction. We identify potent MMIC activity in hepatic stellate cells and liver sinusoidal endothelial cells. MMIC is unlikely exclusive to the liver, as spontaneous acceptance of kidney allografts has been reported, although less commonly, probably reflecting variance in MMIC activity. MMCI may represent an important homeostatic mechanism that supports peripheral tolerance, and could be a target for the prevention and treatment of transplant rejection. This study highlights that the graft is actively participant in the equipoise between tolerance and rejection and warrants more attention in the search for tolerance biomarkers.
Keywords: IFN-γ, B7-H1, immune regulation, T effector cell, apoptosis
Introduction
Due to continuous exposure to the antigens of food and microbial from the gut, the liver has acquired extra capacity of inducing immune tolerance, which was first recognized in the 1960s, where 12 out of 55 (23%) transplants of farmer pig livers survived long term without immunosuppression, and the recipients accepted subsequent skin grafts from the liver donors but not third party animals indicating the development of specific tolerance (1). A similar phenomenon was later reported in rats (2) and mice (3). In the early 90’s, Starzl et al. reported 11 cases of tolerance in liver transplant patients who had been withdrawn from immunosuppression due to non-compliance or post-transplant lymphoproliferative disorder (4). In a subsequent trial designed to wean immunosuppression in 95 liver transplant patients, 29% achieved an immunosuppressant free state for an average of 10.8 years (5). As of 2009, there have been 461 documented cases of attempts at weaning immunosuppression in liver transplant recipients world-wide with 22% being completely free of immunosuppression (6). It has been reported that the combined transplantation of a liver and kidney or lung from the same donor protects improves survival of the non-liver graft (7). The oral tolerance is also linked to the liver, as evidenced by the decline in tolerance following the creation of a portocaval shunt (8). The tendency for viral and parasitic infections to chronically persist in the liver (e.g. HBV, HCV and schistosomiasis) is also related to the livers propensity to favor immunological tolerance (9).
To study the involved mechanisms, we developed a liver transplantation model in mice (10) and demonstrated that liver allografts transplanted across full MHC barriers, for instance from C57BL (H2b) to C3H (H2k), are spontaneously accepted (3). It was initially thought that the liver graft may function like the thymus to delete T cell clones that recognize allo-antigens. This turned out to be unlikely because lymphocytes isolated from recipients bearing long-term surviving graft generated vigorous responses to donor antigens in vitro (11). In vivo, adoptive transferred T cells from TCR transgenic mice in liver transplant recipients underwent complete activation despite graft acceptance (12). This paradoxical phenomenon has been described as ‘split’ (11) or ‘operational’ tolerance (6), and has also been shown after liver transplantation in rats (13) and in other experimental models of alloreactivity (14). The detailed mechanisms are not completely understood.
We then turned the focus on the graft, and showed that liver allograft was heavily infiltrated with lymphocytes early post-transplant, which was gradually, but markedly reduced due to enhancement of apoptotic death of the infiltrating lymphocytes (15). We were inspired by the report that liver allograft was acutely rejected in interferon (IFN)-γ knockout (KO) recipients (16), which is beyond our expectation, because IFN-γ, a key inflammatory cytokine produced by T effector (Tef) cells, participates in graft rejection (13). In this study, we analyzed the liver allografts deficient in IFN-γ receptor and revealed that IFN-γ stimulation is required for elimination of the graft infiltrating Tef cells, which is mediated by the graft mesenchymal cells.
IFN-γ produced by allo-reactive Tef cells activates IFN-γ signaling pathways in graft mesenchymal cells, resulting in upregulated expression of B7-H1 which in turn facilitates Tef cell apoptosis. We describe the intrinsic negative feedback loop between graft mesenchymal cells and Tef cells that leads to operational tolerance.
Materials and Methods
Mice
Male C57BL/6 (B6; H-2b), BALB/c (H-2d), C3H (H-2k), IFN-γR1−/− (H-2b) and GFP-Foxp3 mice (H-2b) were purchased from the Jackson Laboratory (Bar Harbor, ME). B7-H1−/− mice were kindly provided by Dr. Lieping Chen (Johns Hopkins University Medical School, Baltimore, MD). DES (H-2k) TCR transgenic mice expressed H-2Kb specific TCR transgene on CD8+ T cells (17). All mice were maintained in a specific pathogen-free animal facility and used under NIH guidelines. (Please see Materials and Methods in Supporting Data for further details.)
Results
Rejection of IFN-γR1−/− liver allografts is associated with heavy graft infiltration of effector T cells
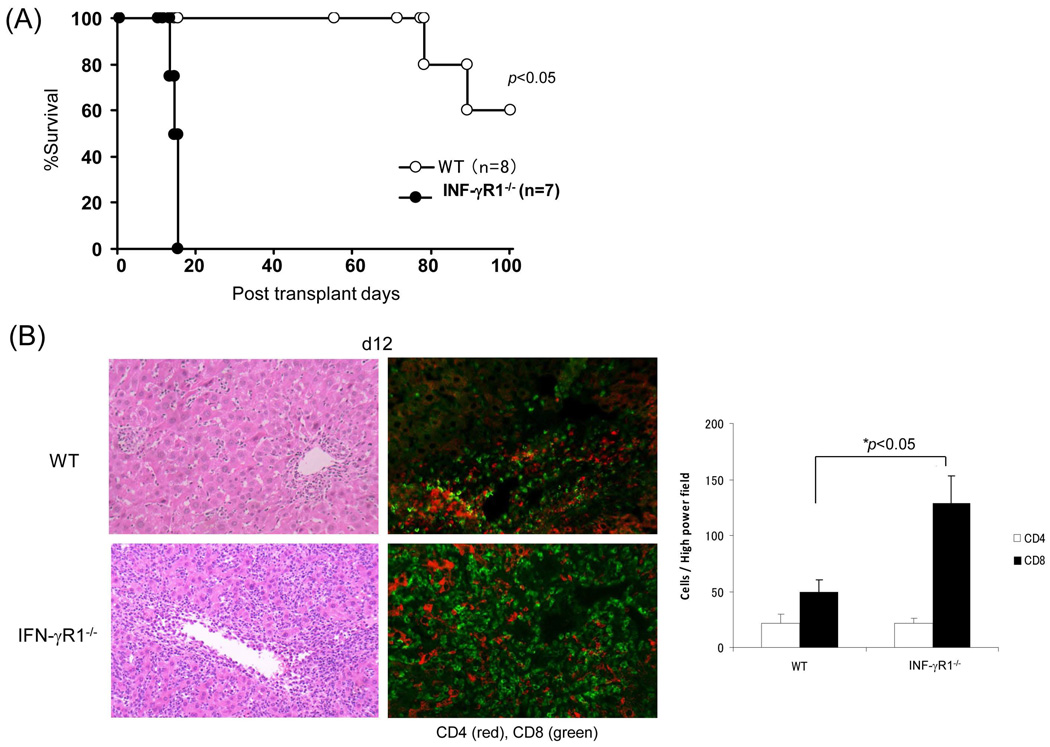
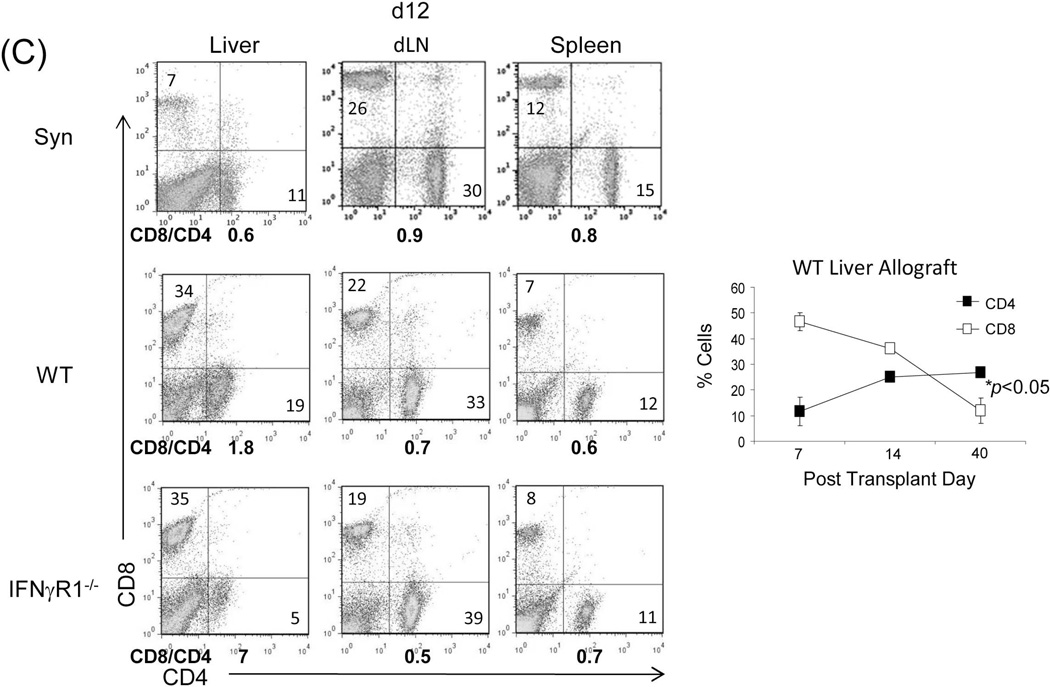
When the liver from IFN-γR1−/− or WT (both B6) mice was transplanted into WT C3H recipients, none of the IFN-γR1−/− liver grafts (n=7) survived > 15 days, whereas all WT grafts (n=8) survived > 80 days (Fig. 1A), indicating that IFN-γR signaling is an absolute requirement for graft acceptance. Histology showed light lymphocyte infiltration in the WT grafts, which was limited to zone I (periportal area), while there was a dense panacinar CD8+ predominant lymphocytic infiltrates in the IFN-γR1−/− grafts (Fig. 1B, left panels). IFN-γR1−/− grafts contained significantly more CD8+ cells than WT controls as documented by immunohistochemistry (Fig. 1B, middle and right panels). Flow analysis showed marked increases in the CD8:CD4 ratio in the IFN-γR1−/−grafts, but not in the draining lymph nodes (dLN) and spleen (Fig. 1C, left panels), suggesting that IFN-γ-mediated immune regulation is mainly orchestrated in the graft. WT allograft recipients were allowed to be followed up long-term and a kinetic analysis of the graft infiltrating cells was notable for a gradual decline in CD8+ T cells with CD4+ T cells becoming predominant (Fig. 1C, right panel).
Figure 1. Liver transplant tolerance is mediated by IFN-γ signaling dependent elimination of effector T cells.
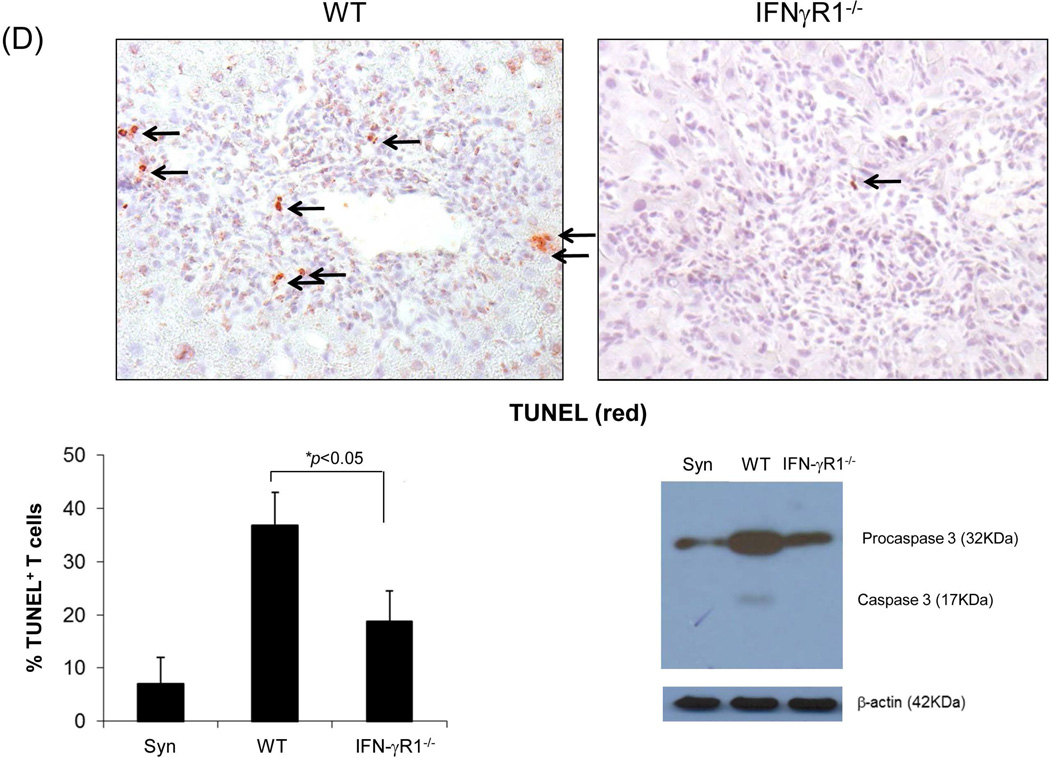
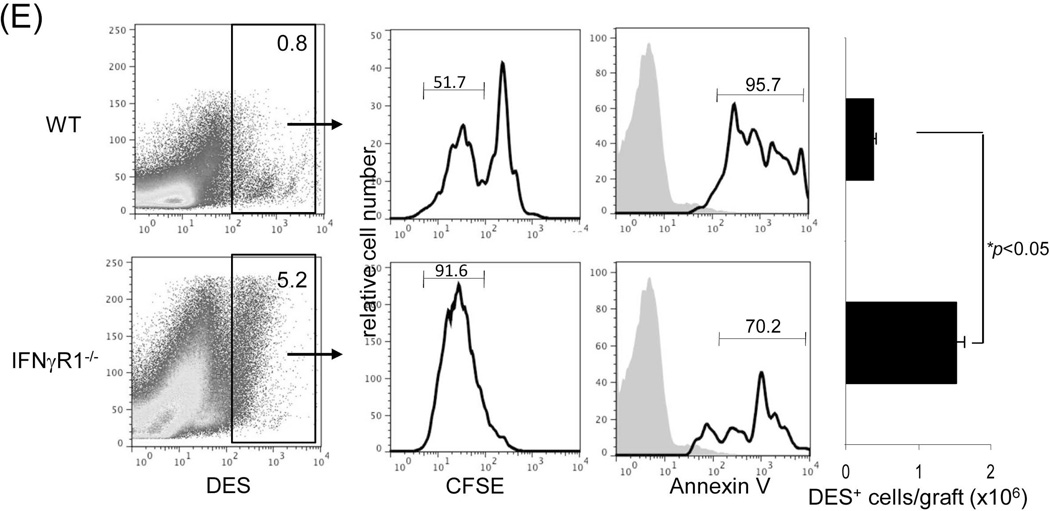
Liver grafts from WT or IFN-γR1−/− mice (both B6, H2b) were transplanted into C3H (H2k) recipients. (A) Survival of the liver allografts was compared between WT (N=8) and IFN-γR1−/− (n=7) groups. (B) Histology of liver allo-transplants on POD12. The graft sections were stained with H&E (left panels) and immunohistochemistry for CD4 (red) & CD8 (green) (middle panels), and the CD4+ and CD8+ cells were counted and expressed as mean cell number/high power field ± SD (n=3) (right panel). (C) Flow analyses of liver graft infiltrating T cells. Left panels: lymphocytes isolated from the WT or IFN-γR1−/− liver allografts on POD 12 were stained for CD4 and CD8. Syngeneic B6 liver grafts served as control (Syn). The number is the percentage of T cell subset in total lymphocytes. CD8/CD4 ratio was calculated and displayed at the bottom. Right panel: Percentage of CD4 and CD8 cells in graft infiltrating lymphocytes isolated from the recipients bearing WT liver allograft for 7, 14 or 40 days, and expressed as mean ± SD (n=3). (D) Apoptotic activity in the liver graft. Upper panels: the sections of liver grafts were stained for TUNEL (red). Lower panels: T cells isolated from the liver graft were stained for TUNEL and analyzed by flow cytometry (left). Protein isolated from these T cells was analyzed for expression of caspase 3 by Western blot (right). (E) Impact of IFN-γ signaling on specific CD8 T cell response. On POD 3, recipients were intravenously given 1.5 × 107CFSE-labeled T cells from DES TCR transgenic mice (H2k). Three days later, lymphocytes were isolated from liver grafts and stained with anti-DES and -annexin V mAbs, and analyzed by flow cytometry gated on DES+ cells and expressed as histograms. The number is the percentage of dividing cells and annexin V+ cells, respectively. The shaded area is isotype control. The absolute number of DES+ cells were calculated based on flow data and expressed as mean/graft ± SD (n=3).
The apoptotic elimination of T effector cells requires intact IFN-γR signaling in the liver allograft
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining of WT and IFN-γR1−/− liver allograft sections demonstrated a marked reduction in apoptotic activity in infiltrating lymphocytes in IFN-γR1−/− liver allografts compared to WT controls (Fig. 1D, upper panels). Flow analysis of graft infiltrating T cells showed a greater frequency of TUNEL+ T cells in the WT group along with higher levels of caspase 3 (western blot) compared to IFN-γR1−/− liver grafts (Fig. 1D, lower panels), highlighting the requirement for IFN-γR signaling in the induction of T cell apoptosis. To examine the donor specific effector T cells, carboxyfluorescein succinimidyl ester (CFSE)-labeled T cells from DES TCR transgenic mice (H-2k) whose CD8+ T cells specifically recognize donor antigen (H-2Kb) were adoptively transferred into liver allograft recipients on post-operative day (POD) 8. The graft infiltrating cells were isolated 3 days later and analyzed for the specific CD8+ T cells by flow cytometry using DES clonotypic mAb (17). Compared to the WT controls, there were markedly more DES+ cells in the IFN-γR1−/− grafts, this was associated with an enhanced proliferative response (CFSE dilution assay) and reduced apoptosis in DES+ CD8+ T cells (annexin V) (Fig. 1E). These findings suggest that IFN-γ signaling in the liver allograft mediates the elimination of effector T cells by inducing apoptosis.
The emergence of endogenous suppressor cells is not dependent on IFN-γ signaling
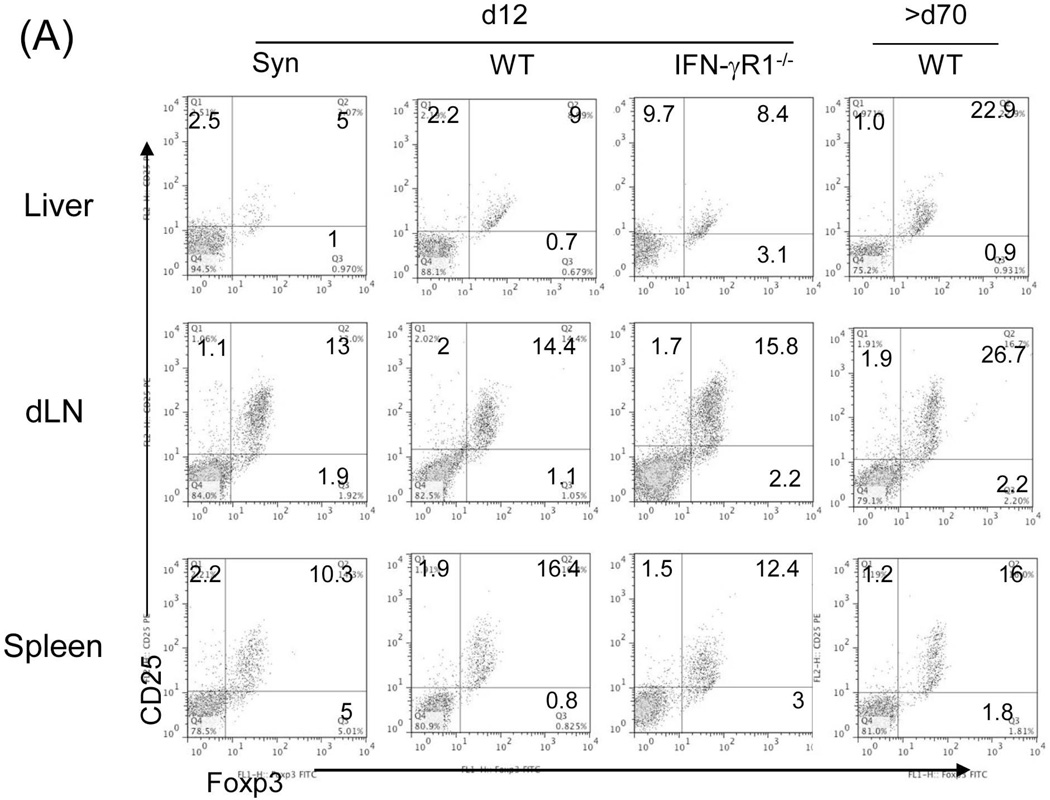
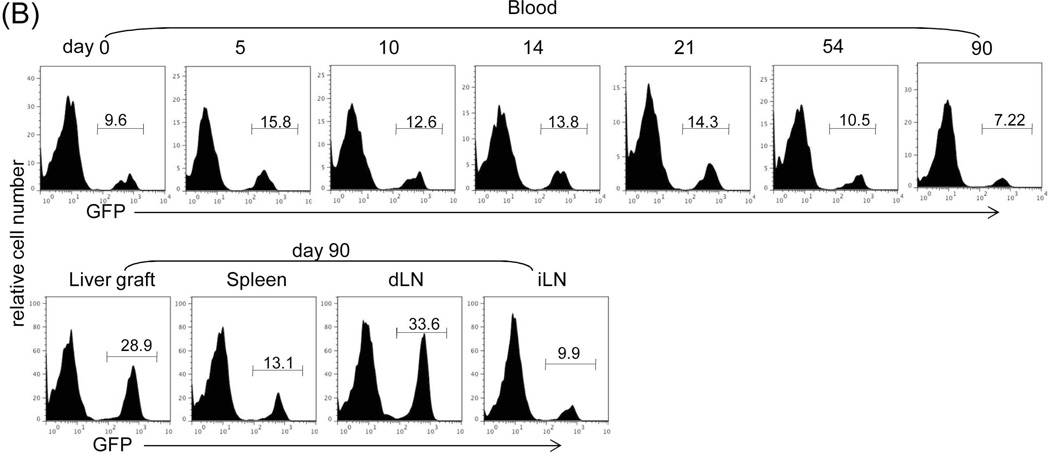
We analyzed the Treg cells by staining for CD4, CD25 and Foxp3 in lymphocytes isolated from the graft, dLN and spleen in liver allograft recipients. The frequency of CD4+CD25+Foxp3+ cells in the WT group was slightly and comparably increased in all tested compartments in WT and IFN-γR1−/− graft recipients on POD 12, compared to the syngeneic controls (Fig. 2A), reflecting the early emergence of Treg cells in response to alloantigen stimulation, which is independent of IFN-γR signaling. In the long-term surviving recipients (only available in WT group), the CD4+CD25+Foxp3+ cells was markedly increased in the graft and dLN, compared to early phase (POD 12) response, but not in the spleen (Fig. 2A), signifying their tendency for local accumulation. The frequency of CD4+CD25+Foxp3+ cells in the long-term surviving syngeneic recipients remained at low levels (data not shown). We monitored Treg cells using green fluorescent protein (GFP)-Foxp3 mice (B6) as recipients of BALB/c liver allograft; these mice express GFP-Foxp3 fusion protein under the control of Foxp3 promoters (18). Kinetic analysis of peripheral blood CD4+ cells revealed a consistent slight increase in GFP+ cells following allogeneic liver (BALB/c) transplantation. In long-term surviving recipients, there was a marked increase in GFP+ cells in grafts and dLN, but not in the spleen and irrelevant LNs (Fig. 2B). We were unable to study the patterns of GFP+ cells in IFN-γR1−/− group since IFN-γR1−/− mice are not available on BALB/c background.
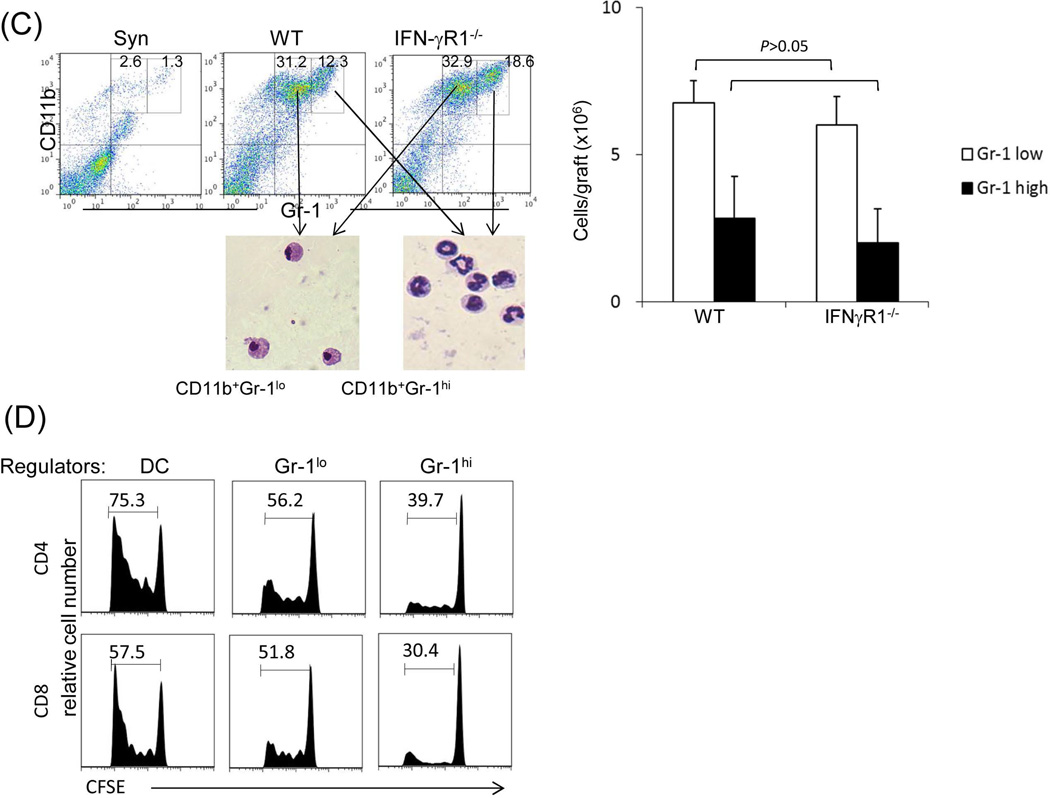
Figure 2. Impact of IFN-γ signaling on the generation of suppressor cells.
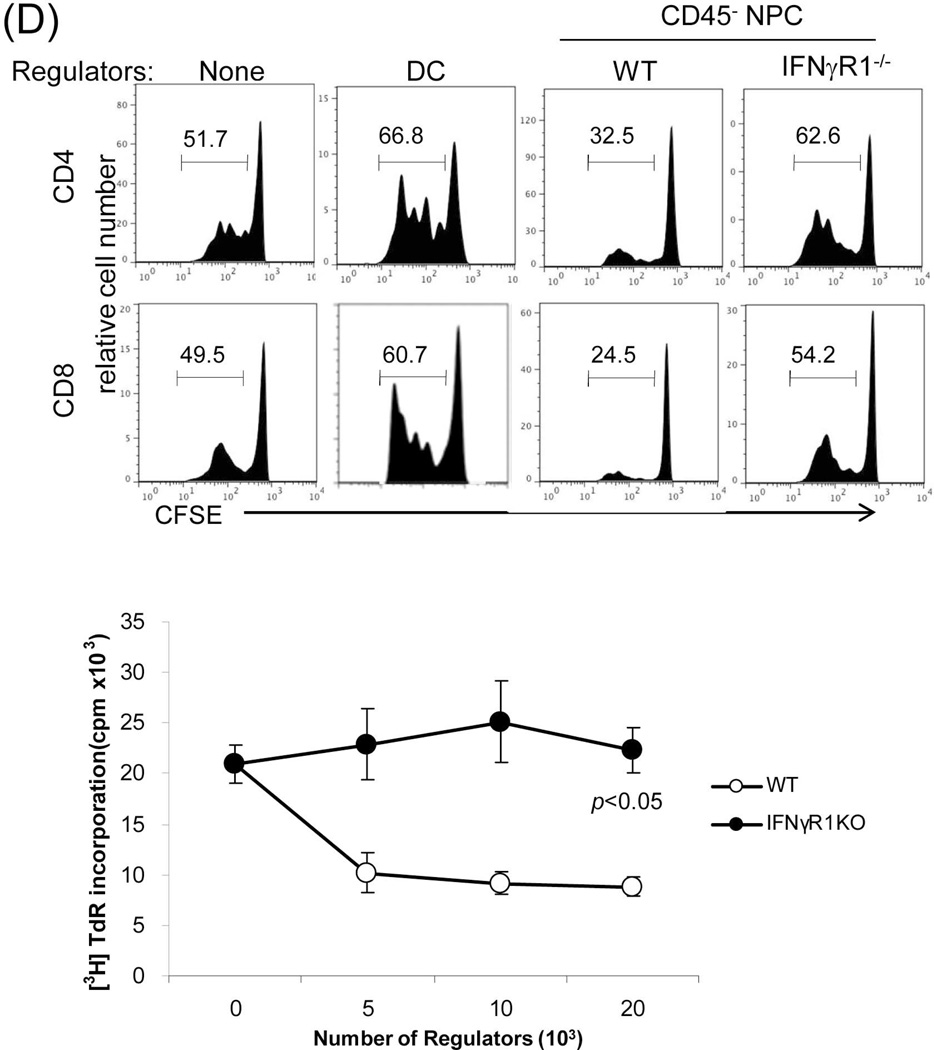
(A) Analysis of Treg cell activity. Lymphocytes isolated from liver graft, draining (d)-LN and spleen in recipients bearing WT or IFN-γR1−/− liver graft for 12 or >70 days (only available in WT group). B6 syngeneic transplantation (Syn) served as control. Cells were triple stained for CD4, CD25 and Foxp3 and analyzed by flow cytometry gated on CD4+ cells. The number is the percentage of positive cells, respectively. (B) Use of GFP-Foxp3 mice: WT BALB/c (H-2d) livers were transplanted into GFP-Foxp3 (H-2b) recipients. Blood was collected on POD 5, 10, 14, 21, 54 and >70 (upper panels). Recipients bearing long-term surviving liver grafts (90 days) were sacrificed and lymphocytes were isolated from liver graft, spleen, d-LN and irrelevant (i)-LN (lower panels). Cells were analyzed by flow cytometry for GFP fluorescence in CD4+ cells, and expressed as histograms. The number is the percentage of GFP positive cells in the CD4+ cell population. The data is representative of three separate experiments. (C) Analysis of myeloid suppressor cells in grafts. The leukocytes isolated from the liver graft on POD 12 were stained for CD11b and Gr-1 for flow analysis. The number is the percentage of CD11b+Gr-1high or Gr-1low cells. The numbers of CD11b+Gr-1low and CD11b+Gr-1high cells per graft were calculated based on flow analysis data and expressed as mean ± SD (n=3). CD11b+Gr-1low and CD11b+Gr-1high cells were sorted out for morphological (Giemsa staining) and the following functional studies. (D) T cell inhibitory activity: The CD11b+Gr-1low and CD11b+Gr-1high cells purified from liver allografts were used as regulators (use of DC as control for comparison), and added at the beginning into a one-way MLR culture where CFSE labeled C3H spleen T cells and B6 DC (at a ratio of 20:1) were used as responders and stimulators, respectively. The regulator: DC ratio was 1:1. An MLR culture without addition of the regulators (none) served as control. The proliferation of T cells was determined by CFSE dilution gated on CD4+ or CD8+ populations. The data is representative of three separate experiments.
The effect of IFN-γ signaling on myeloid-derived suppressor cells (MDSC) was examined by comparing CD11b+Gr-1+ cells isolated from the liver grafts. As shown in Fig. 2C, there were marked and comparable increases in CD11b+GR-1+ cells in WT and IFN-γR1−/− liver allografts, compared to syngeneic controls. They consisted of CD11b+Gr-1hi and CD11b+Gr-1lo populations, and exhibited neutrophil and monocyte morphology, respectively (Fig. 2C). When the purified CD11b+Gr-1hi or CD11b+Gr-1lo cells were added into MLR cultures, both of them either from WT or IFN-γR1−/− grafts markedly suppressed the proliferative responses of CD4+ and CD8 T+ cells (Fig. 2D), suggesting that they are granulocytic (G)-MDSC and monocytic (M)-MDSC, respectively (19). These data indicate that IFN-γ signaling is not required for the enhancement of MDSC in liver grafts. Similar high levels of MDSC were maintained in long-term surviving WT grafts (data not shown).
Graft non-hematopoietic NPC play a crucial role in tolerance induction
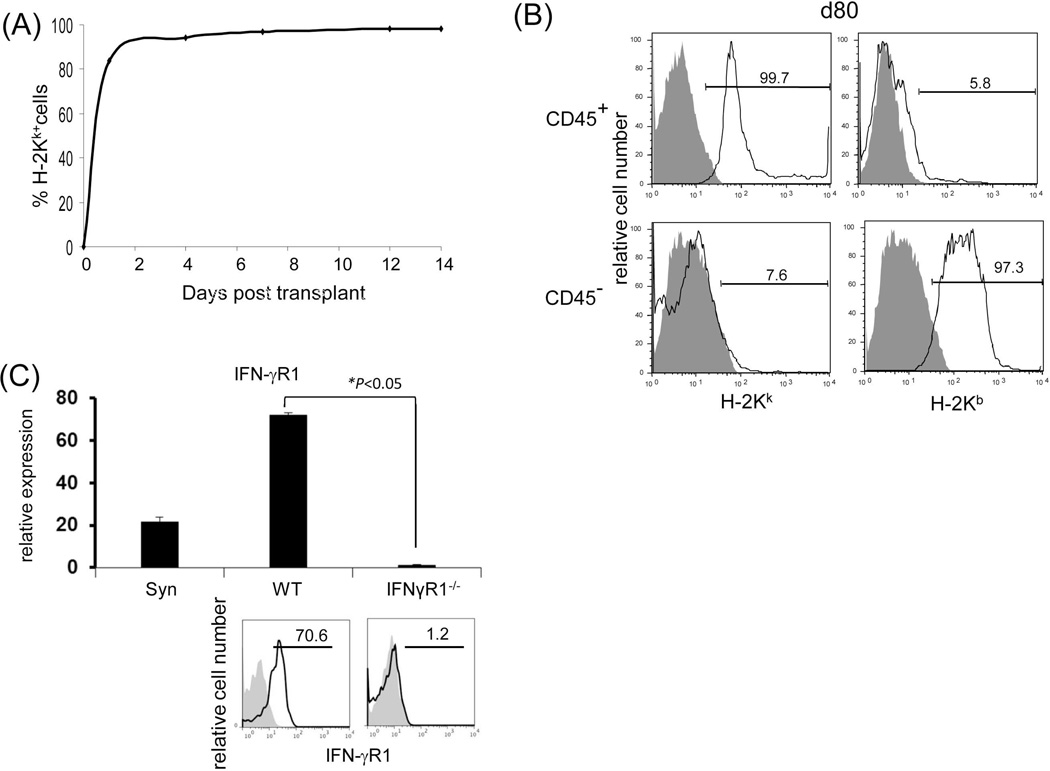
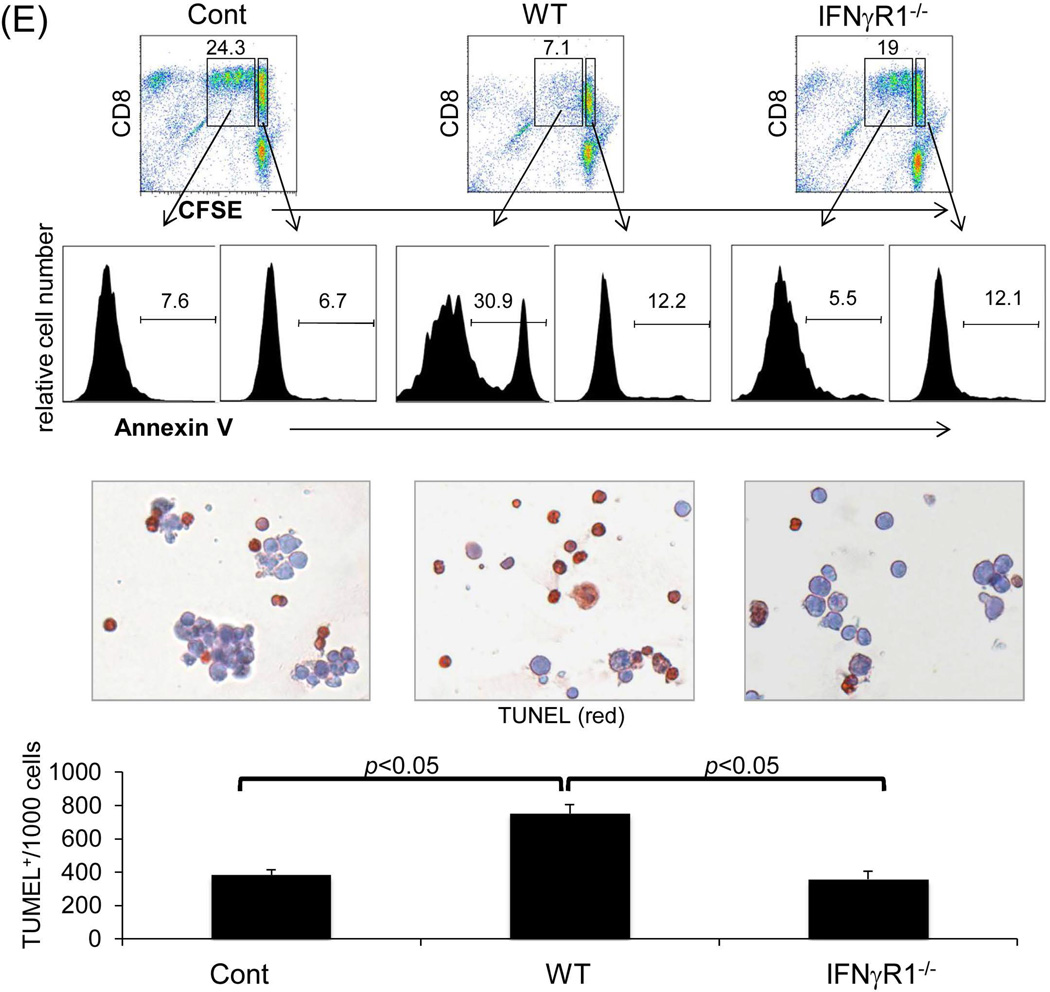
The finding that whole liver allografts are accepted across a complete MHC mismatch whereas hepatocyte allotransplants are universally rejected (20) suggests a critical role for graft NPC in protecting hepatocytes; we therefore focused on studying liver allograft NPC. Kinetic analysis of MHC class I antigen expression on NPC isolated from liver allografts revealed that the graft CD45+ NPC are rapidly replaced by cells of recipient origin following transplantation (Fig. 3A), whereas the CD45− population remain of donor origin even in long-term surviving grafts (Fig. 3B) i.e. in the IFN-γR1−/− graft, CD45+ NPC were quickly replaced by IFN-γR1+/+ cells, while CD45− NPC remained IFN-γR1−/−. This was confirmed by the direct examination of the expression of IFN-γR1 in CD45− NPC isolated from IFN-γR1−/−liver allografts at the both mRNA (q-PCR) and protein levels (flow cytometry) (Fig. 3C). These results suggest that the tolerance is unlikely to be mediated by graft hematopoietic NPC (CD45+), while non-hematopoietic NPC (CD45−) may play an important role in this IFN-γ-dependent transplant tolerance. The function of CD45− NPC isolated from liver grafts was tested by addition into a one-way MLR culture. CD45− NPC from WT grafts inhibited the T cell proliferative response as shown by CFSE dilution and thymidine uptake assays (Fig. 3D). The inhibition of T cell proliferative response was not attributed to overcrowded cell culture because the addition of equivalent number of dendritic cells (DC) showed no inhibitory effect. CD45− NPC from IFN-γR1−/−grafts largely lost their inhibitory activity (Fig. 3D), indicating that their immunosuppressive activity requires an intact IFN-γ signaling. The direct evidence of liver NPC capable of inducing Tef cell apoptosis was obtained by addition of CD45− liver NPC isolated from the WT or IFN-γR−/− mice into the MLR cultures in which the CFSE-labeled H2b specific CD8 T cells (H2k, from DES TCR Transgenic mice) were elicited by B6 DC. Addition of WT NPC was associated with marked inhibition of T cell proliferation, but increase in apoptosis (higher annexin V expression in dividing CD8 specific T cells and more TUNEL+ cells,) while these changes were markedly attenuated in IFN-γR−/− group (Fig. 3E), indicating dependence of IFN-γ signaling pathway.
Figure 3. Graft non-hematopoietic NPC regulate immune response through IFN-γ signaling.
(A) Graft hematopoietic NPC promptly became of recipient origin. Liver NPC were isolated at various time points following transplantation of B6 (H-2b) livers into C3H (H-2k) recipients (syngeneic transplantation served as control). They were stained for CD45, donor (H-2Kb) and recipient (H-2Kk) MHC class I, and analyzed by flow cytometry. The data was expressed as percentage of H-2Kk+ cells in CD45+population. (B) Graft non-hematopoietic NPC remain of graft origin. NPC isolated from long-term surviving liver grafts were analyzed by flow cytometry for CD45, H-2Kb and H-2Kk, and expressed as histograms. Shaded area is isotype control. The number is the percentage of positive cells in the CD45+ or CD45− populations, respectively. The data are representative of three separate experiments. (C) Expression of IFN-γR1 on graft non-hematopoietic NPC. CD45− NPC purified from liver grafts (POD 12) were tested for expression of IFN-γR1 in mRNA (q-PCR) (upper panel, n=3) and protein levels (flow cytometry, lower panels). The number is the percentage of IFN-γR1 positive cells in the CD45− cell population. (D) Graft non-hematopoietic cell-mediated T cell inhibition is dependent on intact IFN-γ signaling. CFSE labeled C3H spleen T cells were cultured with B6 DC at a ratio of 20:1 for 3 days. CD45− cells from WT or IFN-γR1−/− liver allografts (use of DC as regulators for comparison) were added at the beginning into the culture at a regulator: DC ratio of 1:1. A MLR culture without addition of the regulator cells (none) served as control. The proliferation of T cells was determined by CFSE dilution gated on CD4+ or CD8+ populations (the number is the percentage of dividing cells, upper panels) or by thymidine uptake (n=3, lower panel). (E) Graft CD45− NPC induce T effector cell apoptosis via IFN-γ pathway in vitro. CFSE-labeled H2b specific CD8+ T cells from DES TCR transgenic mice (H2k) were cultured with B6 DC at a ratio of 20:1 for 3 days. CD45− NPC isolated from WT or IFN-γR1−/− liver allograft were added at the beginning into the culture at a DC:NPC=1:1. Addition of same number of B6 DC served as control (Cont). The cells were double stained with anti-CD8 and -annexin V mAbs, and analyzed by flow cytometry for the proliferation of specific T cells (CFSE dilution) gated on CD8+ population (upper panels). The number is percentage of dividing cells. Expression of annexin V was analyzed on dividing or non-diving CD8+ cells. Cell suspensions were stained with TUNEL and examined under a microscope. The number is percentage of annexin V+ cells in CD8+ cells. The cell suspensions were also stained with TUNEL (red) and examined under microscope, and counted, expressing as mean TUNEL+ cell number (in 1000 counted cells) ± SD. The data are representative of two separated experiments.
B7-H1 expression on graft non-hematopoietic NPC is critical in liver transplant tolerance
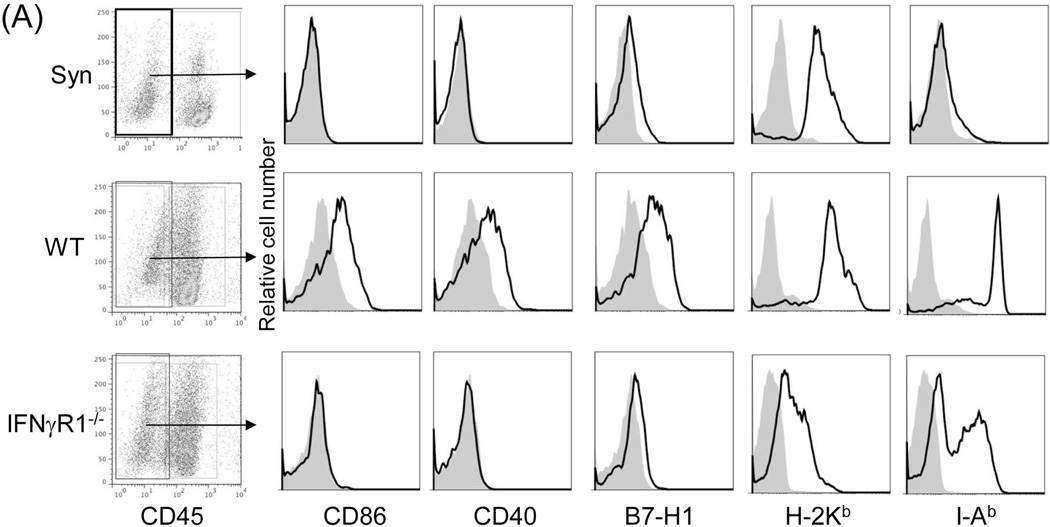
To identify the downstream effector mechanism of IFN-γ signaling, we analyzed the expression of key surface molecules on CD45−NPC isolated from WT and IFN-γR1−/−liver allografts by flow cytometry. As expected, CD45− NPC from IFN-γR1−/−grafts expressed lower MHC class I and II, CD40, CD86 and B7-H1 (Fig. 4A), reflecting the diverse effect of IFN-γ on the immunologically relevant genes (21). We noted the reduced B7-H1 expression in IFN-γR1−/−graft, which could account for its rejection via ligation with its receptor PD-1 predominantly expressed on activated T cells, resulting in apoptosis (22). This is supported by our previous study showing that the B7-H1−/− liver allograft was acutely rejected in WT recipients (23), despite the fact that graft hematopoietic NPC were promptly replaced by B7-H1+/+ cells of recipient origin.
Figure 4. B7-H1 mediates the immune regulatory function of graft non-hematopoietic NPC.
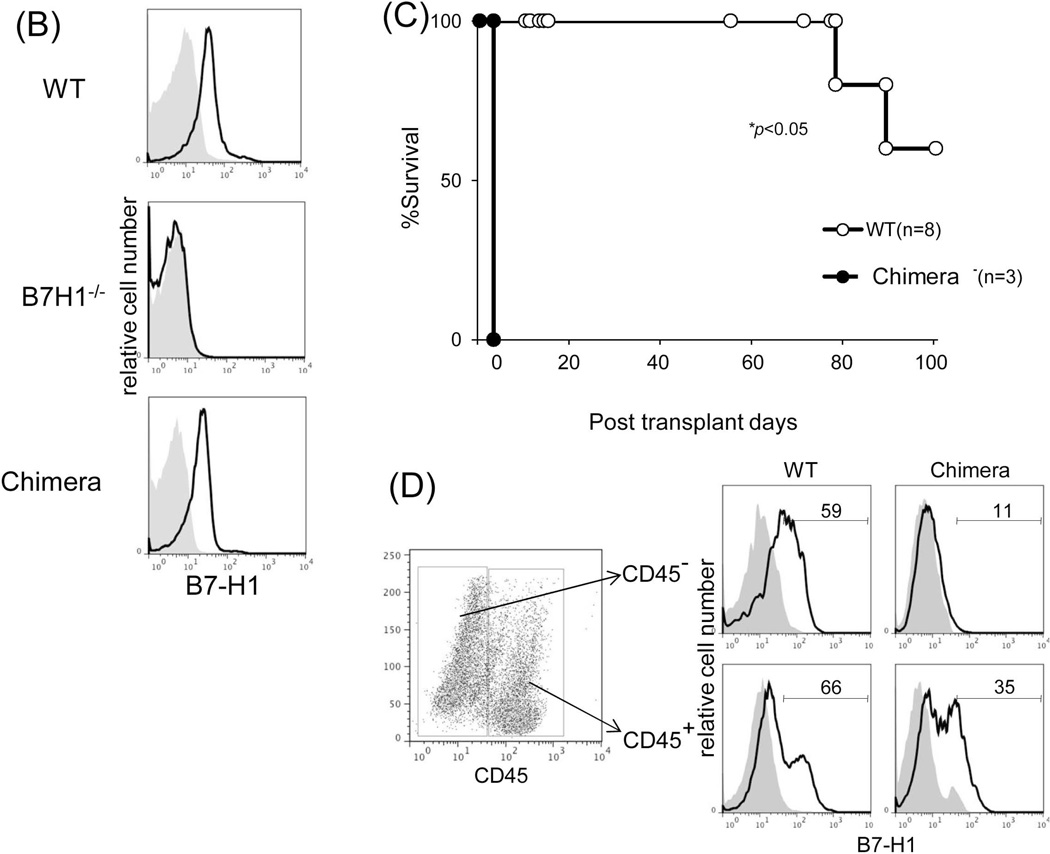
(A) Expression of key surface molecules on graft non-hematopoietic NPC. Expression of CD40, CD86, B7-H1, MHC class I and II was analyzed by flow cytometry gated on CD45− NPC that were isolated from WT or IFN-γR1−/− liver allograft on POD 12, expressed as histograms. Syngeneic graft (Syn) served as control. The shaded area is isotype control. (B) Establishment of chimeras. Transplantation of BM from WT B6 into lethally irradiated B7-H1−/− mice as described in the Methods section. Five weeks later, chimeric status was examined by determining expression of B7-H1 on peripheral blood cells by flow cytometry, expressed as histograms. Blood from naïve WT B6 or B7-H1−/− mice served as controls. (C) Liver transplant tolerance requires B7-H1 expression on graft non-hematopoietic NPC. The liver from chimeric (n=3) or WT mice (n=8) was transplanted into WT C3H recipients. Graft survival was compared. (D) Expression of B7-H1 on NPC of liver allografts. NPC were isolated from chimeric or WT liver allografts on POD 5. Expression of B7-H1 was analyzed gated on CD45+ and CD45− cells, respectively, expressed as histograms. The shaded area is isotype control. The number is the percentage of positive cells. The data are representative of three separate experiments.
To precisely analyze the role of B7-H1, we generated chimeric mice by transplanting bone marrow (BM) (B6) into lethally irradiated B7-H1−/− (B6) mice. Five weeks later, following confirmation of chimerism (Fig. 4B), the chimeric liver was transplanted into WT allogeneic recipients. All chimeric grafts (n=3) were acutely rejected (Fig. 4C). The chimeric status was reexamined by isolating graft NPC and staining for CD45 and B7-H1. CD45+ NPC became B7-H1+, while CD45− NPC remained B7-H1−/− (Fig. 4D). These data indicate that B7-H1 on graft non-hematopoietic NPC mediates liver transplant tolerance. The role of B7-H1 expression on graft hematopoietic NPC was further excluded by achievement of long-term survival of WT C3H liver that was transplanted into B7-H1−/− recipients (B6), in which the CD45+ cells in the graft quickly became B7-H1−/−, while the CD45− cells remained B7-H1+/+ (data not shown).
Identification of the graft cell components that contribute to immune regulation
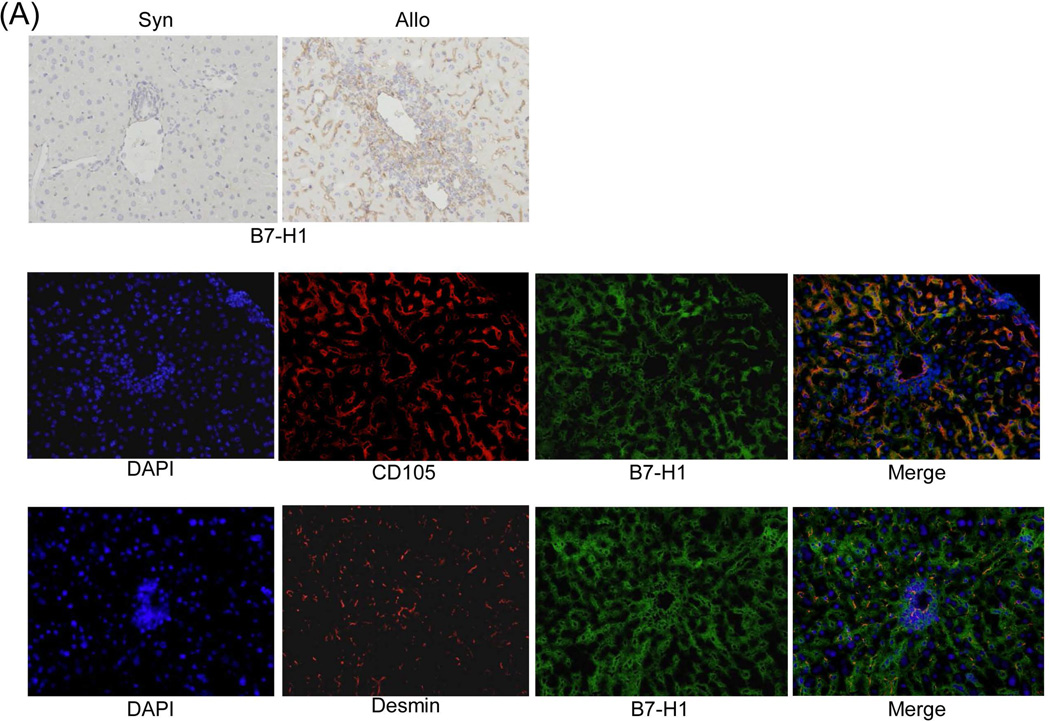
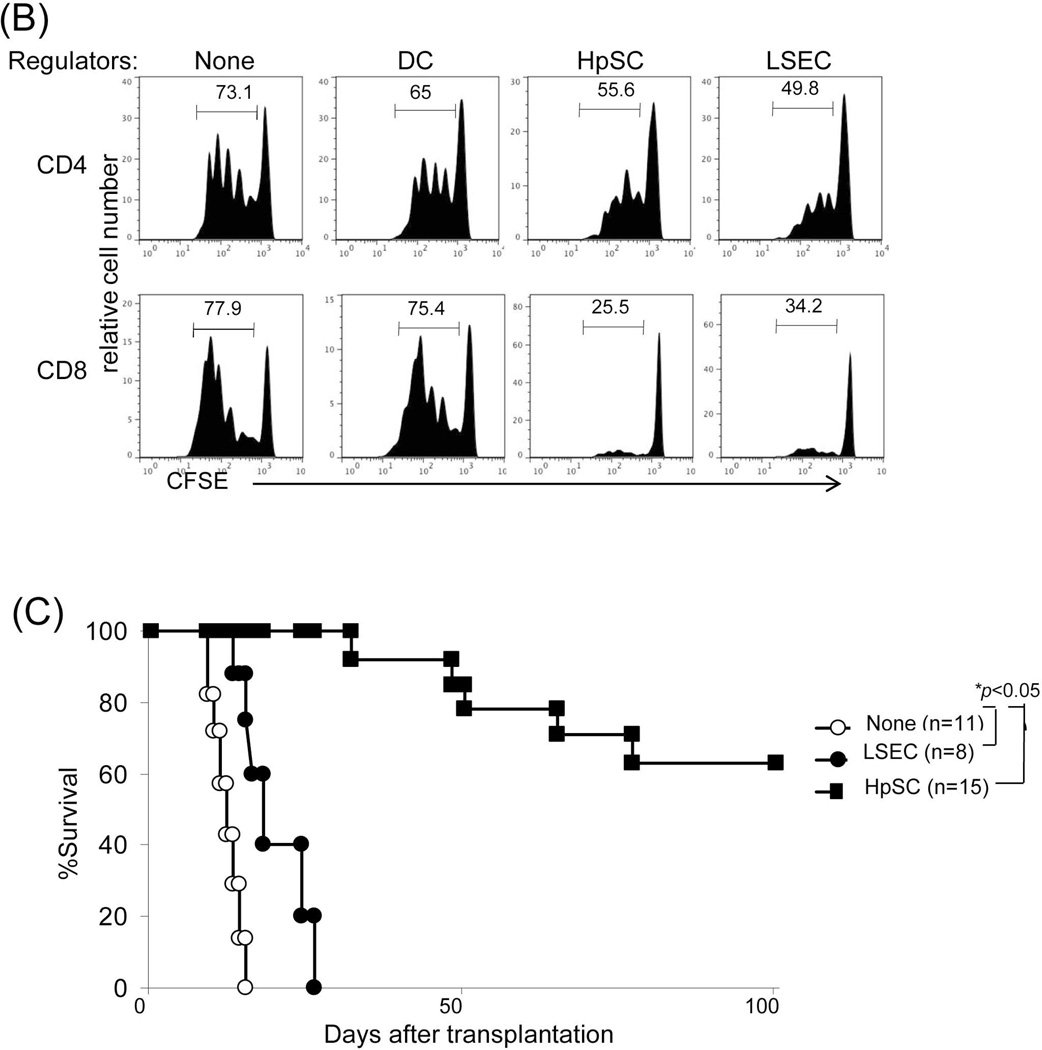
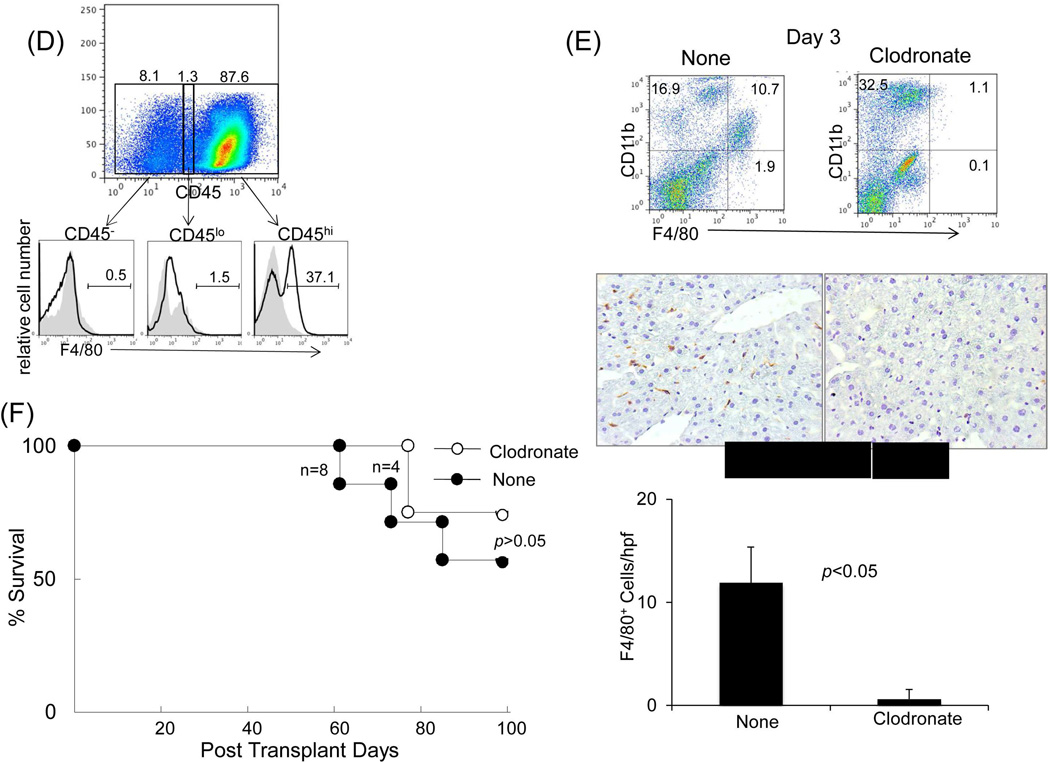
Immunohistochemistry demonstrated a paucity of B7-H1 expression in isografts, whereas allografts were notable for strong B7-H1 expression, which was predominantly localized in the sinusoids and periportal areas (Fig. 5A, upper panels). Staining with cell specific mAbs showed that many of the B7-H1+ cells were CD105+ [marker for liver sinusoidal endothelial cells (LSEC)] (24) or desmin+ [marker for hepatic stellate cells (HpSC)] (25) (Fig. 5A, middle and lower panels), suggesting a potential immunoregulatory function of these populations. To assess their immune regulatory function, LSEC and HpSC were isolated from liver allografts. Addition of either LSEC or HpSC into an MLR culture markedly inhibited the T cell proliferative response, particularly CD8+ T cells (Fig. 5B). The immune regulatory function of these cells was verified in vivo by co-transplanting isolated LSEC or HpSC with islet allografts into diabetic recipients. Co-transplantation of either LSEC or HpSC prolonged islet allograft survival (both p<0.05, compared to islet allografts alone), and HpSC were far superior to LSEC in protecting islet allografts from rejection (Fig. 5C). We considered liver CD45− cells may contain sessile Kupffer cells (KC) which are not derived from BM (26). To examine this, expression of F4/80 on graft NPC was analyzed by flow analysis. Almost all F4/80+ cells were CD45hi, while very few were CD45− or CD45lo (Fig 5D). Actually, approximately half of the CD45− NPC were endothelial cells (43% CD146+ and ~60% CD31+) (Supporting data Fig.1). To test the function of KC in vivo, liver donor mice were treated with clodronate liposomes to destroy KC in the liver (27,28), which was documented by flow and immunohistochemical analyses (Fig. E). The liver grafts deficient in KC remained spontaneously accepted by allogeneic recipients (Fig. 5F), suggesting that sessile KC are not critical in induction of live transplant tolerance.
Figure 5. Identification of immunoregulatory components in graft non-hematopoietic NPC.
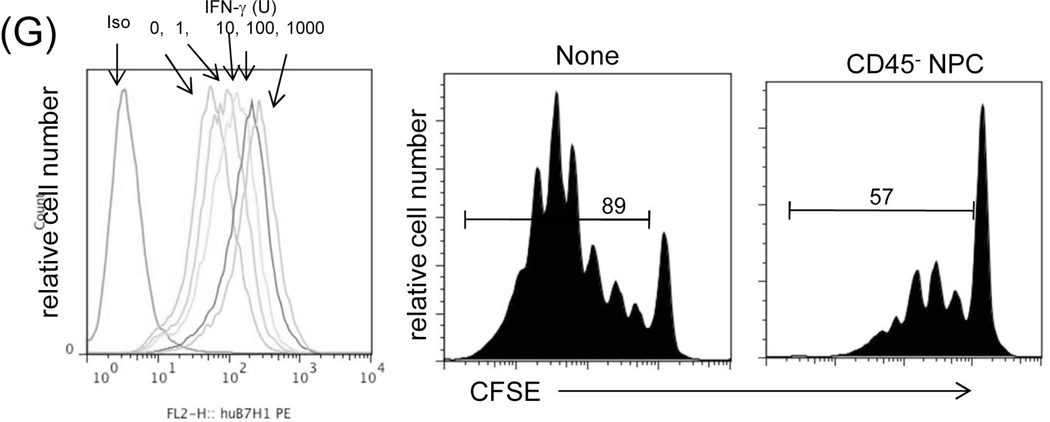
(A) Identification of B7-H1 positive cells in the liver graft. Liver grafts were harvested on POD 8 following transplantation of B6 liver into C3H recipients (syngeneic grafts served as controls). The cryostat sections were single stained for B7-H1 (brown) (upper panels) or triple double fluorescently stained for B7-H1(green) and CD105 or Desmin (red) and DAPI (blue), and examined under microscope. (B) Graft LSEC and HpSC inhibit T cell proliferative response. LSEC and HpSC isolated from the liver allografts were used as the regulators (DC served as controls), and added at the beginning into a one-way MLR culture in which CFSE-labeled C3H T cells were stimulated by B6 DC (T : DC = 20:1) at a regulator: stimulator ratio of 1:1. T cell proliferative response was determined by CFSE dilution. The number is the percentage of dividing cells. The data are representative of three separate experiments. (C) LSEC and HpSC prolong survival of co-transplanted islet allografts. 3×105 isolated LSEC or HpSC were mixed with 300 pancreatic islets isolated from BALB/c mice and transplanted under the renal capsule of B6 diabetic recipients. Recipients transplanted with islet allografts alone were used as controls. Survival of islet allografts was determined by monitoring blood glucose as described in the Methods section. (D) No Kupffer cells are identified in CD45− and CD45low cell populations. NPC isolated from the WT liver allograft were double stained for CD45 and F4/80 and analyzed for expression of F4/80 in CD45hi, CD45lo and CD45− populations, expressing as histograms. The number is percentage of F4/80+ cells. (E) Depletion of graft sessile Kupffer cells. Liver donor mice (B6) were i.v. injected with 200 µl of clodronate liposomes 3 days before transplanted into C3H recipients. The depletion of Kupffer cells were documented by absence of F4/80+ cells by flow analysis and liver sections by immunochemical staining. (F) Kupffer cells are not critical in liver transplant tolerance. Survival of normal and Kupffer cell-depleted liver allografts was comparable (p>0.05). (G) Human liver non-hematopoietic NPC inhibit T cell response. Expression of B7-H1 on human liver non-hematopoietic NPC is enhanced by IFN-γ stimulation. Human liver CD45− NPC were incubated with IFN-γ at various concentrations for 18 hours and stained with anti-hB7-H1 mAb for flow analysis [demonstrated as histogram (upper panel)]. Human liver CD45− NPC were added at the beginning into a CFSE-labeled PBMC-derived T cell culture (at a ratio of 1:10), the proliferation of T cells was elicited by anti-CD3/CD28 beads. No addition of NPC served as control (None). T cell proliferative response was determined by CFSE dilution assay gated in CD3+ cells (lower panels). The data are representative of three separate experiments.
Human liver non-hematopoietic NPC inhibit T cell response
Liver NPC were isolated from human liver tissue, and the CD45− NPC were purified by magnetic bead separation, and exposed to different concentrations of IFN-γ. Exposure to IFN-γ enhanced surface expression of B7-H1 in a dose dependent manner (Fig. 5G, left panel), suggesting induction of B7-H1 expression in an inflammatory environment. To test their inhibitory activity, the isolated CD45− NPC were added into a one-way MLR culture where proliferation of CFSE labeled T cells was elicited by anti-CD3/CD28 beads, resulting in marked inhibition of the T cell proliferative responses (Fig. 5G, right panels).
Discussion
In investigating why liver transplant tolerance requires IFN-γ, we find that IFN-γ stimulation promotes apoptosis of infiltrating Tef cells tipping the immunological balance towards tolerance. An equivalent increase in Treg cells and MDSC was seen in the rejection (IFN-γR1−/−) and tolerant (WT) groups. These results suggest that the enhancement of Treg cells and MDSC alone in the early post-transplant period is not sufficient to induce liver transplant tolerance, whereas, eliminating the alloreactive effector T cells is critical. This is in agreement with the recent studies. Administration of Treg cells alone is inadequate to induce transplant tolerance, whereas Treg administration coupled with T cell depletion does (29). The requirement of IFN-γ, a Tef cell-produced cytokine suggests that the alloreactive response is absolutely required for the induction of transplant tolerance. In other word, rejection triggers the process of the operational tolerance. This may explain why agents targeting T cell activation (e.g. calcineurin inhibitors) can abrogate the establishment of tolerance in the murine organ transplant models (30,31).
We show that the IFN-γ-dependent liver transplant tolerance is mediated by graft CD45− NPC, because the CD45+ cells in IFN-γR−/− liver allografts are rapidly replaced by recipient IFN-γR+/+ cells after transplantation, while CD45− NPC remain IFN-γR−/−. This is supported by the data that CD45− NPC from WT liver allograft markedly suppress T cell proliferative response in vitro, but not in IFN-γR1−/− group. We demonstrate that the LSEC and HpSC isolated from liver allografts are immunosuppressive. In particular HpSC, the important mesenchymal cells in the liver, are capable of protecting islet allografts with >60% achieving survival >100 days. These observations along with previous reports by us and others suggest that graft mesenchymal cells are major players in immunoregulation. Thus, LSEC isolated from normal livers inhibit T cell proliferation in vitro by cross-presenting antigen to CD8+ T cells (32,33). We reported that quiescent HpSC are not immunosuppressive, but become suppressive following activation by inflammatory stimuli (25,34). Co-transplantation of activated (but not quiescent) HpSC markedly prolongs the survival of islet allografts (34,35). T cell inhibition by HpSC is not MHC-restricted, since HpSC from third party strain can also effectively inhibit T cell response elicited by alloantigen in vitro (25). We note that co-transplantation with HpSC from donor or third party strain fails to prolong islet allograft survival due to rejection of the HpSC themselves (34). However, HpSC in liver grafts are also of donor origin, but are not rejected. The discordant results could be explained by the existence of other tissue NPC including LSEC to form a protection network in liver allograft. We considered that CD45− cells could contain sessile Kupffer cells (KC) which are not derived from BM (26), and their role in tolerance has been controversial (36,37). The present study demonstrates that the depletion of sessile KC in liver allografts does not break the tolerance, suggesting that KC are unlikely to be critical.
Our data suggest that expression of B7-H1 on graft non-hematopoietic NPC is a key molecule in mediating liver transplant tolerance. Thus, CD45− NPC from IFN-γR1−/− grafts do not express B7-H1, whereas the counterparts in WT grafts express high B7-H1. The liver allografts from chimeras (in which the B7-H1−/− phenotype is limited to the CD45−NPC) are acutely rejected. This is also supported by the rejection of B7-H1−/− liver allografts in WT recipients despite the prompt repopulation of the hematopoietic NPC of recipient (B7-H1+/+) origin (23). The B7-H1 expressed on graft hematopoietic NPC seems not crucial in induction of the tolerance because we showed that WT liver allografts are accepted by B7-H1−/− recipients where the graft hematopoietic cells promptly become B7-H1−/−. The underlying mechanisms are not completely understood. We note that, in contrast to broader expression of B7-H1 (PDL-1), the expression of B7-DC (PDL-2) which shares the receptor PD-1 with B7-H1, is restricted to DC, macrophages and B cells, B7-DC often shows potent co-stimulatory activity (38). The co-inhibitory activity of B7-H1 on hematopoietic cells may be compromised by competitions of B7-DC and other co-stimulatory molecules. The parenchymal cells (hepatocytes) may not actively participate in B7-H1-mediated immune tolerance because they do not express B7-H1 (Fig. 5A).
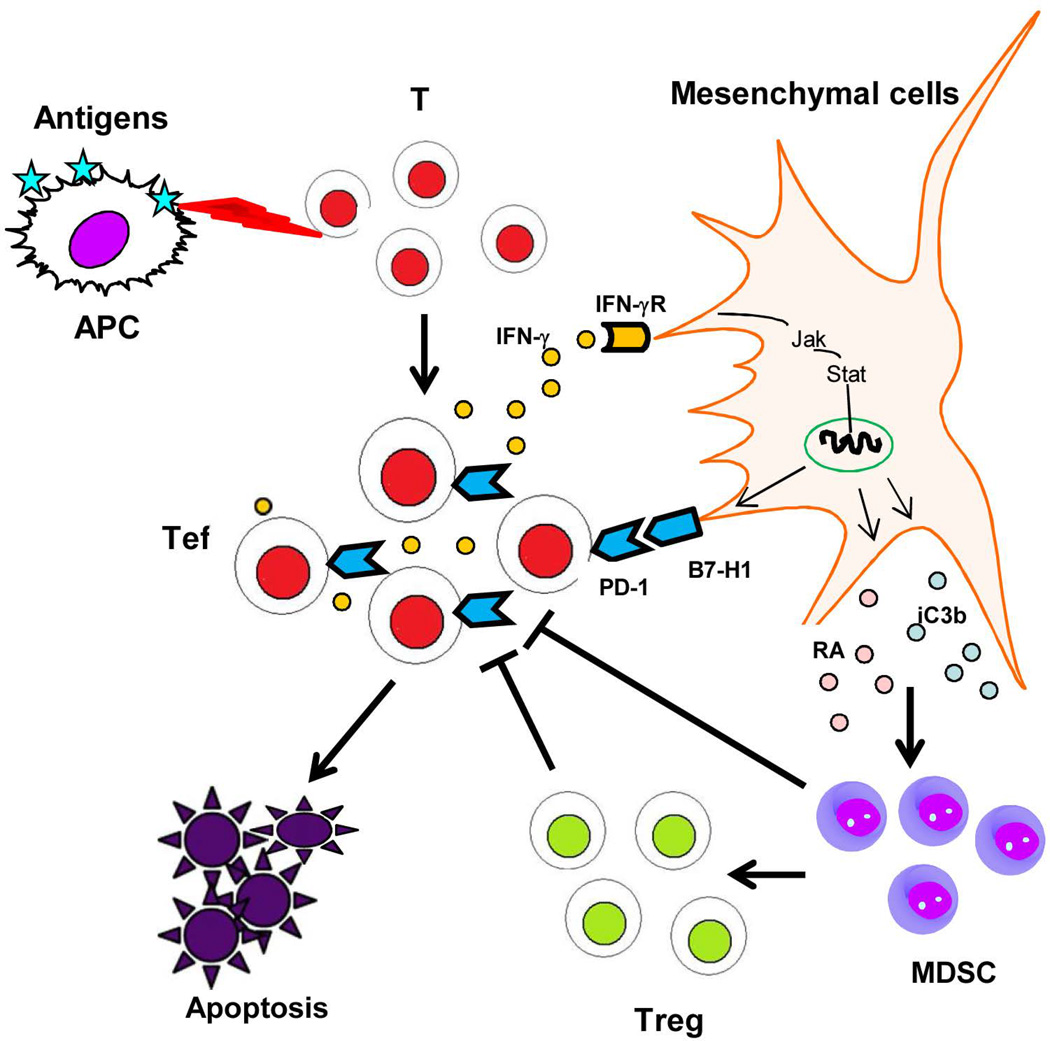
We describe a novel mesenchyme-mediated immune control (MMIC) mechanism in the liver allograft. The scenario is that IFN-γ originating from the alloreactive Tef cells stimulates graft mesenchymal cells resulting in upregulated B7-H1 expression that in turn facilitates the death of Tef cells. MMIC activity represents an intrinsic negative feedback loop between graft mesenchymal cells and Tef cells leading to establishment of operational tolerance. The allograft is not a passive player in the face of the host immune attack, rather it is capable of generating a robust counter response in the form of MMIC. The reliance of MMIC on IFN-γ indicates that an initial pro-inflammatory microenvironment is a precondition for the induction of the tolerance. MMIC is mediated by graft mesenchymal cells, which differs from graft versus host disease (GVHD) that is mediated by graft lymphocytes, resulting in extensive host tissue damage (39). MMIC is unlikely a phenomenon exclusive to the liver, since stellate cells exist in the kidney and pancreas, and endothelial cells are ubiquitous (40,41). Spontaneous acceptance of kidney allografts has been reported, although less commonly than the liver (42). The varying incidence of spontaneous tolerance among organs may reflect the variance in their MMIC capability. The vigorous MMIC exhibited by the liver could be explained by the presence of abundant stellate cells in addition to endothelial cells. The potent MMIC activity of the liver is unsurprising given its constant exposure to external antigens from gut through the portal circulation. Moreover, HpSC display many of the characteristic features of MSC, with many experts arguing that HpSC are liver-resident mesenchymal stem cells (MSC) (43). MSC show immune modulatory property which is mediated by B7-H1 signaling (44). Therefore, based on the extensive distribution of tissue resident MSC, we propose that MMIC may represent a wider homeostatic mechanism that supports peripheral tolerance. We hypothesize that self-reactive T eclls (low affinity) that have escaped central tolerance mechanisms (45) encounter cognate antigens in the periphery, react, and produce inflammatory cytokines that trigger tissue mesenchymal cells, resulting in expression of B7-H1 to induce apoptosis in autoimmune Tef cells, and production of soluble factors, such iC3b and retinoic acid, to promote generation of MDSC and Treg cells (46–48) (Fig. 6). Peripheral tolerance is thus established.
Figure 6. Schematic of the role mesnechyme-mediated immune control (MMIC) mechanism in peripheral T cell tolerance.
Self-reactive T cells escaped from the negative selections in thymus are activated by the cognate antigens in the periphery, produce IFN-γ, and trigger the IFN-γ signaling pathways in mesenchymal cells, resulting in B7-H1 expression to induce Tef cell apoptosis, and production of the soluble factors to promote generation of MDSC and Treg cells. Autoreactive T cell response is thus inhibited in periphery.
Many transplant tolerance induction protocols have been tested in pilot clinical studies. All of them have targeted manipulations of host immunity (49). Similarly, almost all of the studies designed to identify transplant tolerance biomarkers have focused on host immunity (50,51). Our data strongly suggest a critical role for the graft in emergence of rejection or tolerance. More attentions should be paid to donor and graft in searching for tolerance biomarkers. Solid organ transplantation has been successful for decades, but the outcomes of cell transplantation (such as islet and hepatocyte transplants) remain disappointing. The lack of appropriate mesenchymal cells to mediate MMIC activity may contribute to the poor outcomes of cell transplants. The improvement of islet transplants by co-transplantation with HpSC (34,35), testicular Sertoli cells (52) or MSC (53) represent endeavors to harness the MMIC activity.
The enhancement of MMIC is an attractive therapeutic strategy for transplant rejection and autoimmune diseases.
Supplementary Material
Expression of CD11c, CD146, CD31, F4/80 was analyzed by flow cytometry gated on CD45− NPC isolated from WT liver allografts on POD 12, expression as histograms. The shade area is isotype control. The data is representative of two separate experiments.
ACKNOWLEDGMENT
This work has been supported by funds from the U.S. National Institute of Health Grant DK084192 awarded to L.L. and AI090468 awarded to SQ. We thank Dr. Liping Chen (John Hopkins University Medical School) for providing B7-H1 knockout mice, Dr. Xiaokang Li for providing help to identify B7-H1 positive cells by immunohistochemistry, Paul Pavicic for help to generate chimeric mice, Kathleen Brown for performing some immunohistochemistry.
Abbreviations
- BM
Bone marrow
- CFSE
carboxyfluorescein succinimidyl ester
- CTL
cytotoxic T lymphocyte
- dLN
draining lymph nodes (dLN)
- GFP
green fluorescent protein
- HpSC
hepatic stellate cells
- IFN-γR
interferon-gamma receptor
- KO
knockout
- KC
Kupffer cells
- LSEC
liver sinusoid endothelial cells
- MMIC
mesenchyme- mediated immune control
- MLR
mixed leukocyte reaction
- MDSC
myeloid-derived suppressor cells
- MSC
mesnechymal stem cells
- NPC
non-parenchymal cells
- POD
post-operative day
- q-PCR
quantitative-polymerase chain reaction
- Tef
T effector
- Treg
T regulatory
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- WT
wild type
References
- 1.Calne RY, et al. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;233:472–476. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 2.Kamada N, Brons G, Davies HS. Fully allogeneic liver grafting in rats induces a state of systemic nonreactivity to donor transplantation antigens. Transplantation. 1980;29:429–431. doi: 10.1097/00007890-198005000-00021. [DOI] [PubMed] [Google Scholar]
- 3.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: Tolerance and donor cell chimerism. Hepatology. 1994;19:916–924. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reyes J, et al. Frequent achievement of a drug-free state after orthotopic liver transplantation. Transplant. Proc. 1993;25:3315–3319. [PMC free article] [PubMed] [Google Scholar]
- 5.Mazariegos GV, et al. Weaning of immunosuppression in liver transplant recipients. Transplantation. 1997;63:243–249. doi: 10.1097/00007890-199701270-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orlando G, Soker S, Wood K. Operational tolerance after liver transplantation. J Hepatology. 2009;50:1247–1257. doi: 10.1016/j.jhep.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Benseler V, McCaughan GW, Schlitt HJ, Bishop GA, Bowen DG, Bertolino P. The liver: a special case in transplantation tolerance. Semin Liver Dis. 2007;27:94–213. doi: 10.1055/s-2007-979471. [DOI] [PubMed] [Google Scholar]
- 8.Callery MP, Kamei T, Flye MW. The effect of portocaval shunt on delayed-hypersensitivity responses following antigen feeding. J Surg Res. 1989;46:391–394. doi: 10.1016/0022-4804(89)90208-4. [DOI] [PubMed] [Google Scholar]
- 9.Crispe IN. Immune tolerance in liver disease. Hepatology. 2014;60:2109–2117. doi: 10.1002/hep.27254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qian S, Fung JJ, Demetris AJ, Ildstad S, Starzl TE. Orthotopic liver transplantation in mice. Transplantation. 1991;52:562–564. doi: 10.1097/00007890-199109000-00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahmen U, et al. Split tolerance induced by orthotopic liver transplantation in mice. Transplantation. 1994;58:1–8. doi: 10.1097/00007890-199407000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein I, Crispe IN. Complete differentiation of CD8+ T cells activated locally within the transplanted liver. J Exp Med. 2006;203:437–447. doi: 10.1084/jem.20051775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ring GH, Saleem S, Dai Z, Hassan AT, Konieczny BT, Baddoura FK, Lakkis FG. Interferon-γ is necessary for initiating the acute rejection of major histocompatibility complex class II-disparate skin allografts. Transplantation. 1999;67:1362–1371. doi: 10.1097/00007890-199905270-00012. [DOI] [PubMed] [Google Scholar]
- 14.Sprent J, Hurd M, Schaefer M, Heath W. Split tolerance in spleen chimeras. J. Immunol. 1995;154:1198–1206. [PubMed] [Google Scholar]
- 15.Qian S, et al. Apoptosis within spontaneously accepted mouse liver allografts: evidence for deletion of cytotoxic T cells and implications for tolerance induction. J. Immunol. 1997;158:4654–4661. [PMC free article] [PubMed] [Google Scholar]
- 16.Mele TS, et al. IFN-γ is an Absolute Requirement for Spontaneous Acceptance of Liver Allografts. Am J Transplant. 2003;3:942–951. doi: 10.1034/j.1600-6143.2003.00153.x. [DOI] [PubMed] [Google Scholar]
- 17.Hua C, Boyer C, Guimezanes A, Albert F, Schmitt-Verhulst A M. Analysis of T cell activation requirements with the use of alloantigens or an anticlonotypic monoclonal antibody. J. Immunol. 1986;136:1927–1936. [PubMed] [Google Scholar]
- 18.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 19.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J. Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bumgardner GL, et al. A functional model of hepatocyte transplantation for in vivo immunologic studies. Transplantation. 1998;65:53–61. doi: 10.1097/00007890-199801150-00011. [DOI] [PubMed] [Google Scholar]
- 21.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-γ: an overview of signals, mechanisms and functions. J Leu Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. (2004). [DOI] [PubMed] [Google Scholar]
- 22.Keir ME, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morita M, et al. PD1/B7H1 interaction contributes to the spontaneous acceptance of liver allograft. Am. J. Transplant. 2010;10:40–46. doi: 10.1111/j.1600-6143.2009.02859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie G, et al. Hedgehog signalling regulates liver sinusoidal endothelial cell capillarisation. Gut. 2012;62:299–309. doi: 10.1136/gutjnl-2011-301494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu M-C, et al. Inhibition of T Cell Responses by Hepatic Stellate Cells via B7-H1 Mediated T Cell Apoptosis. Hepatology. 2004;40:1312–1321. doi: 10.1002/hep.20488. [DOI] [PubMed] [Google Scholar]
- 26.Klein I, et al. Kupffer cell heterogeneity: functional properties of bone marrow-derived and sessile hepatic macrophages. Blood. 2007;110:4077–4085. doi: 10.1182/blood-2007-02-073841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stienstra R, et al. Kupffer cells promote hepatic steatosis via interleukin-1β-dependent suppression of peroxisome proliferator-activated receptor α activity. Hepatology. 2010;51:511–522. doi: 10.1002/hep.23337. [DOI] [PubMed] [Google Scholar]
- 28.Ikarashi, et al. Distinct development and functions of resident and recruited liver Kupffer cells/macrophages. J Leu Biol. 2013;94:1325–1337. doi: 10.1189/jlb.0313144. [DOI] [PubMed] [Google Scholar]
- 29.Lee K, Nguyen V, Lee K-M, Kang S-M, Tang Q. Attenuation of Donor-Reactive T Cells Allows Effective Control of Allograft Rejection Using Regulatory T Cell Therapy. Am. J. Transplant. 2014;14:27–38. doi: 10.1111/ajt.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, Li XC, Zheng XX, Wells AD, Turka LA, Strom TB. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat Med. 1999;5:1298–1302. doi: 10.1038/15256. [DOI] [PubMed] [Google Scholar]
- 31.Wekerle T, Kurtz J, Bigenzahn S, Takeuchi Y, Sykes M. Mechanisms of transplant tolerance induction using costimulatory blockade. Curr Opin Immunol. 2002;14:592–600. doi: 10.1016/s0952-7915(02)00378-3. [DOI] [PubMed] [Google Scholar]
- 32.von Oppen N, et al. Systemic antigen cross-presented by liver sinusoidal endothelial cells induces liver-specific CD8 T-cell retention and tolerization. Hepatology. 2009;49:1664–1672. doi: 10.1002/hep.22795. [DOI] [PubMed] [Google Scholar]
- 33.Schurich A, et al. Dynamic Regulation of CD8 T Cell Tolerance Induction by Liver Sinusoidal Endothelial Cells. J. Immunol. 2010;184:4107–4114. doi: 10.4049/jimmunol.0902580. [DOI] [PubMed] [Google Scholar]
- 34.Chen C-H, et al. In vivo immune modulatory activity of mouse hepatic stellate cells. Hepatology. 2006;44:1171–1181. doi: 10.1002/hep.21379. [DOI] [PubMed] [Google Scholar]
- 35.Yang HR, et al. Mechanistic insights into immunomodulation by hepatic stellate cells in mice: a critical role of interferon-γ signaling. Hepatology. 2009;50:1981–1991. doi: 10.1002/hep.23202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.You Q, Cheng L, Kedl RM, Ju C. Mechanism of T cell tolerance induction by murine hepatic Kupffer Cells. Hepatology. 2008;48:978–990. doi: 10.1002/hep.22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knoshita M, et al. Characterization of F4/80-positive Kupffer cell subsetd by their function and phenotype in mice. J Heptol. 2010;53:903–910. doi: 10.1016/j.jhep.2010.04.037. [DOI] [PubMed] [Google Scholar]
- 38.Tseng SY, wt al. B7-DC, a new dentritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spiryda L, Laufer MR, Soiffer RJ, Antin JA. Graft-versus-host disease of the vulva and/or vagina: Diagnosis and treatment. Bio. Blood Marrow Transplant. 2003;9:760–765. doi: 10.1016/j.bbmt.2003.08.001. (2003). [DOI] [PubMed] [Google Scholar]
- 40.Nagy NE, Holven KB, Roos N, Senoo H, Kojima N, Norum KR, Blomhoff R. Storage of vitamin A in extrahepatic stellate cells in normal rats. J. Lipid. Res. 1997;38:645–658. [PubMed] [Google Scholar]
- 41.Shimizu K, Kobayashi M, Tahara J, Shiratori K. Cytokines and peroxisome. proliferator-activated receptor gamma ligand regulate phagocytosis by pancreatic stellate cells. Gastroenterol. 2005;128:2105–2104. doi: 10.1053/j.gastro.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 42.Russell PS, Chase CM, Colvin RB, Plate JM. Kidney transplants in mice. An analysis of the immune status of mice bearing long-term, H-2 incompatible transplants. J. Exp. Med. 1978;147:1449–1468. doi: 10.1084/jem.147.5.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kordes C, Sawiitza I, Gotze S, Haussinger D. Hepatic stellate cells support hematopoiesis and are liver-resident mesenchymal stem cells. Cell. Physiol. Biochem. 2013;31:290–304. doi: 10.1159/000343368. [DOI] [PubMed] [Google Scholar]
- 44.Wang H, et al. Requirement of B7-H1 in mesenchymal stem cells for immune tolerance to cardiac allografts in combination therapy with rapamycin. Transpl immunol. 2014;31:65–74. doi: 10.1016/j.trim.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 45.Hogquisi KA, Baldwin TA, Jemeson SC. Central tolerance: learning self-control in the thymus. Nat Rev Immunol. 2005;5:772–782. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- 46.Hsieh C-C, et al. The role of complement component 3 (C3) in differentiation of myeloid-derived suppressor cells. Blood. 2013;121:1760–1768. doi: 10.1182/blood-2012-06-440214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhatt S, et al. All-trans retinoic acid induces arginase-1 and inducible nitric Oxide synthase-producing dendritic cells with T cell inhibitory function. J Immunol. 2014;192:5098–5108. doi: 10.4049/jimmunol.1303073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chou H-S, et al. Myeloid-derived suppressor cells protect islet transplants via B7-H1 mediated enhancement of T regulatory cells. Transplantation. 2012;93:272–282. doi: 10.1097/TP.0b013e31823ffd39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scandling JD, Busque S, Shizuri JA, Engleman EG, Strober S. Induced immune tolerance for kidney transplantation. N. Engl. J. Med. 2011;365:1359–1360. doi: 10.1056/NEJMc1107841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sagoo P, et al. Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest. 2010;120:1848–1861. doi: 10.1172/JCI39922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Londono M-C, Danger D, Giral M, Soulillou J-P, Sanchez-Fueyo A, Brouard S. A need for biomarkers of operational tolerance in liver and kidney transplantation. Am J Transpl. 2012;12:1370–1377. doi: 10.1111/j.1600-6143.2012.04035.x. [DOI] [PubMed] [Google Scholar]
- 52.Suarez-Pinzon W, Korbutt GS, Power R, Hooton J, Rajotte RV, Rabinovitch A. Testicular sertoli cells protect islet beta-cells from autoimmune destruction in NOD mice by a transforming growth factor-beta-1-dependent mechanism. Diabetes. 2000;49:1810–1818. doi: 10.2337/diabetes.49.11.1810. [DOI] [PubMed] [Google Scholar]
- 53.Figliuzzi M, et al. Bone marrow-derived mesenchymal stem cells improve islet graft function in diabetic rats. Transplant Proc. 2009;41:1797–1800. doi: 10.1016/j.transproceed.2008.11.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expression of CD11c, CD146, CD31, F4/80 was analyzed by flow cytometry gated on CD45− NPC isolated from WT liver allografts on POD 12, expression as histograms. The shade area is isotype control. The data is representative of two separate experiments.