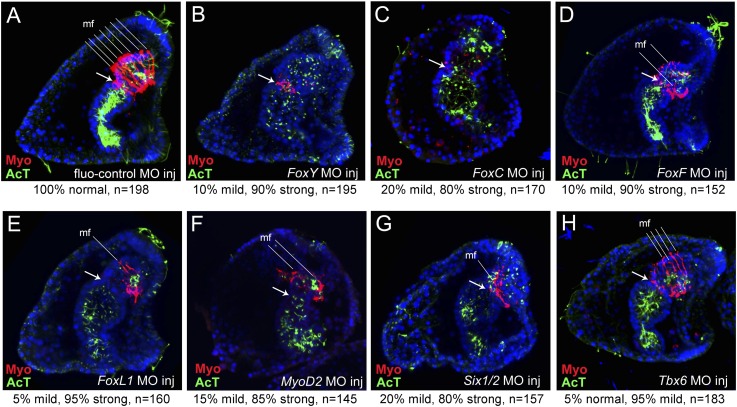
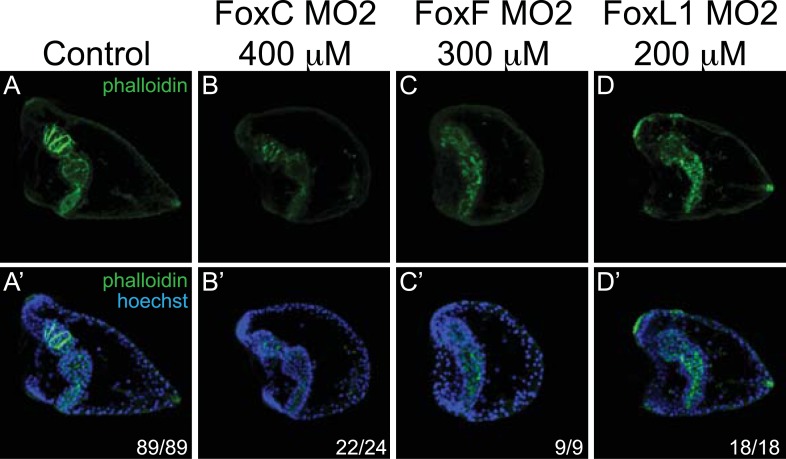
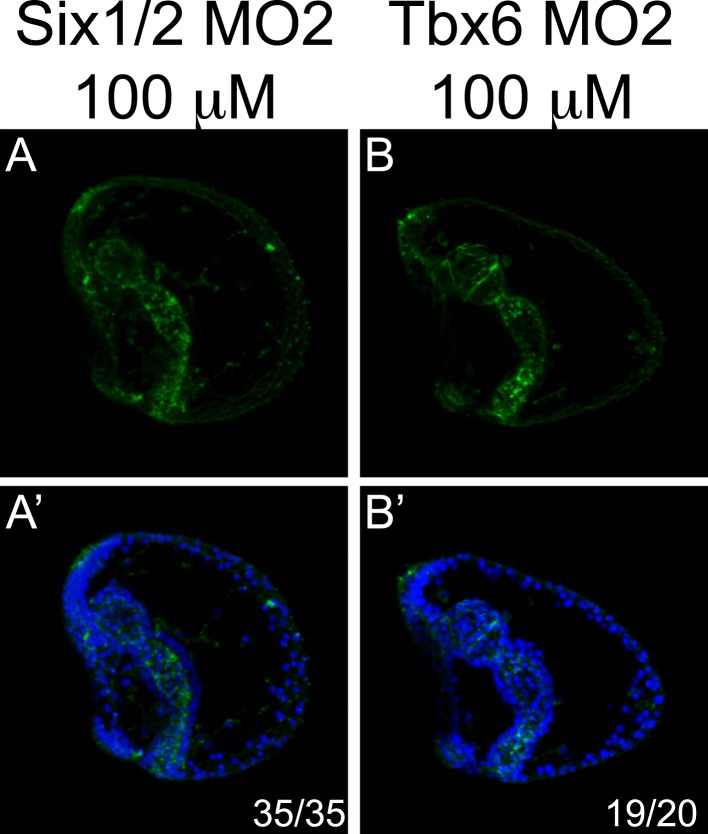
Figure 4. MHC protein detected by immunostaining after perturbation of putative myogenic regulators.
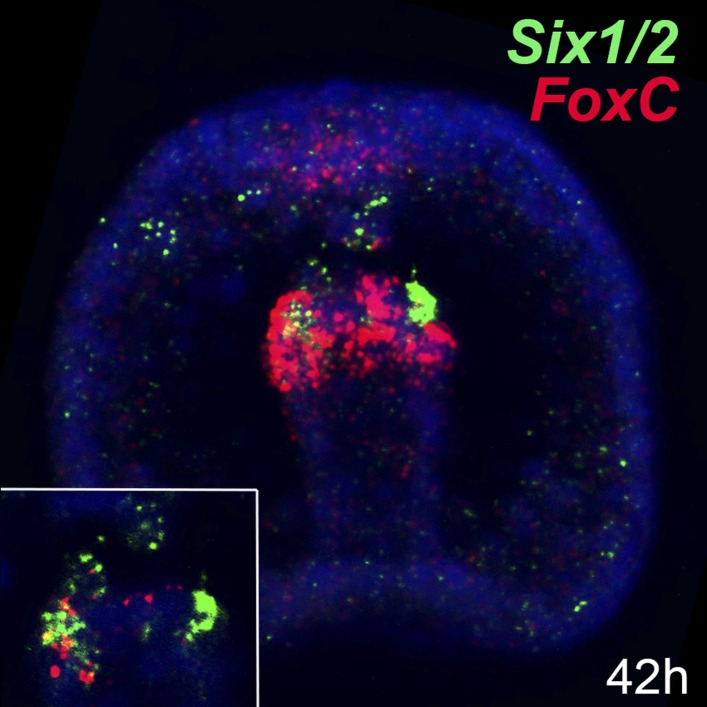
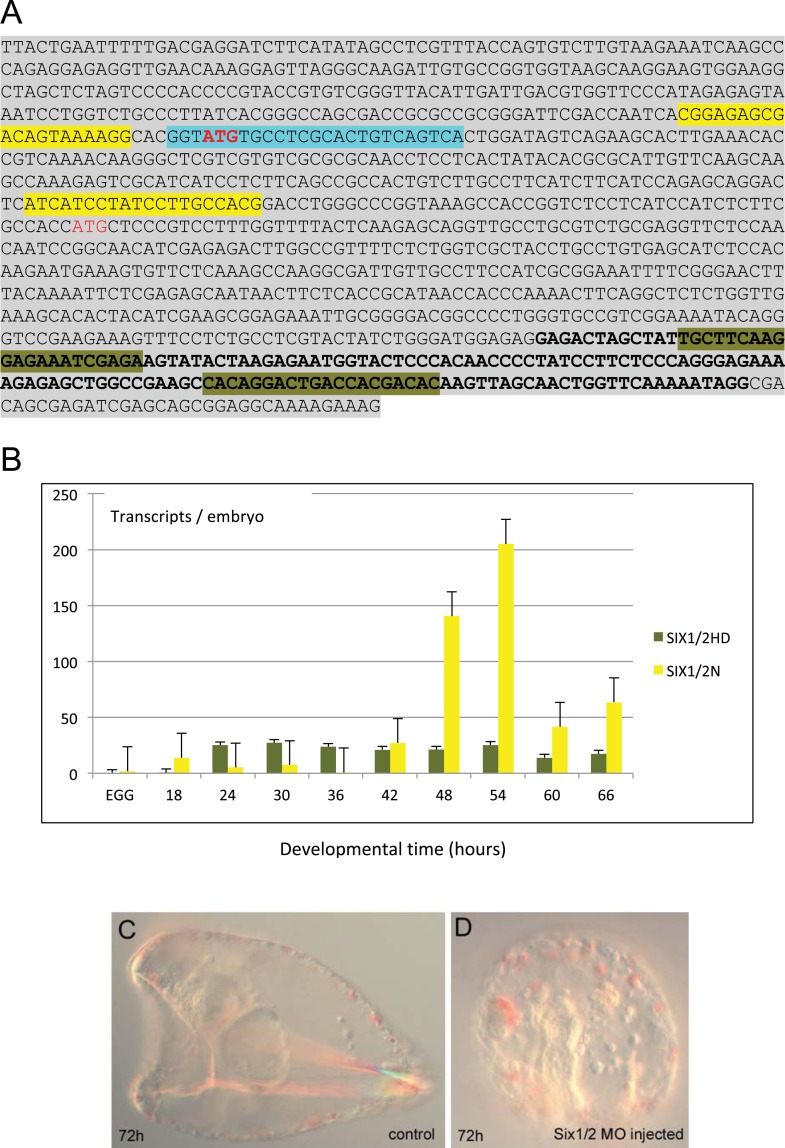
MHC protein localization was tested by immunostaining in fluo-control MO-injected pluteus larvae (72 hr) (A) and in embryos of the same age injected with MOs against FoxY (B), FoxC (C), FoxF (D), FoxL1 (E), MyoD2 (F), Six1/2N (G), and Tbx6 (H) (for MO injection controls see also Materials and methods and Figure 2—figure supplement 2). The ciliary band and gut internal cilia were stained by immunohistochemistry with an anti-acetylated tubulin antibody. Each picture is a stack of merged confocal Z sections with MHC in red and acetylated tubulin in green. Nuclei were labeled blue with DAPI. All embryos are seen in lateral view with the oral side on the right. White arrows indicate the position of cardiac sphincters. White lines indicate muscle fibers (mf). Below each panel, statistics of muscle fiber phenotype observed are reported as normal (6–7 mf), mild (4–5 mf), or strong (0–2 mf). A co-expression analysis of Six1/2 and FoxC is reported in Figure 4—figure supplement 1. Analysis of the temporal expression profile of two distinct Six1/2 isoforms and visualization of pigmentation after perturbing Six1/2N isoform are reported in Figure 4—figure supplement 2.