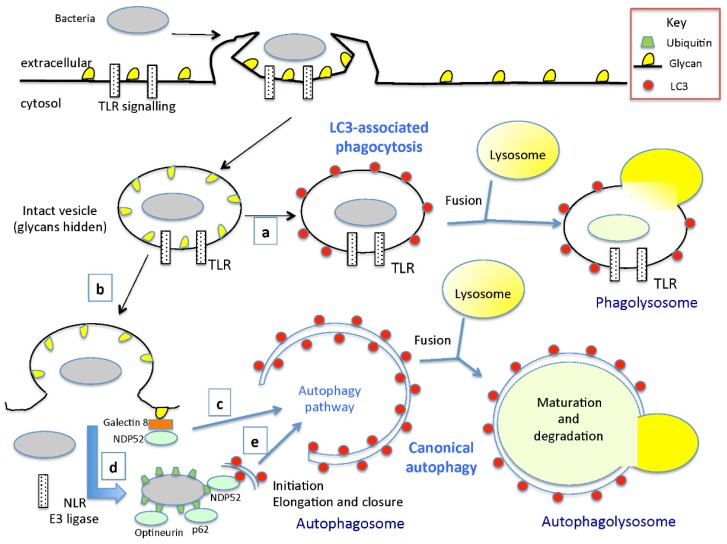
Figure 2.
A simplified overview (a modified schema from [38,50,51] of LC3-associated phagocytosis (LAP) and canonical autophagy. (a) LAP is triggered by Toll-like receptors (TLRs) and other pattern recognition receptors (PRRs) in response to microorganisms, such as bacteria, that are taken up by phagocytosis or that have actively invaded non phagocytic cells. LAP requires a subset of autophagy genes for the labelling of phagosomes with LC3 (Atg8), which promotes their lysosomal delivery and the efficient killing of vesicular pathogens; (b) Damage of the limiting membrane of the pathogen-containing vesicle, either accidental or caused by pathogens attempting to escape from the vesicle, exposes the cytosol to glycans previously hidden inside the vesicle; (c) Cytosol-accessible glycans are detected by galectin-8 (danger receptor), which, by recruiting the cargo receptor NDP52 (nuclear dot protein 52), triggers autophagy; (d) Pathogens having escaped galectin-8-induced autophagy are met by another layer of PRRs in the cytosol such as NLR, and a yet-to-be-identified E3 ubiquitin ligase causes the ubiquitin-coating of invading bacteria. It remains to be established whether this ligase only targets membrane-associated or also free-floating bacteria, whether it is a PRR, and also whether its substrate is of bacterial [52] or host origin such as LRSAM1 [53] or WWP1 [54]; (e) A dominant pathway in the autophagic capture of bacteria such as Salmonella relies on tagging bacteria with a poly-ubiquitin coat, which is then bound by three apparently non-redundant ubiquitin binding autophagy adaptors, NDP52, p62 and optineurin [55,56,57,58]. As pointed out by Mostowy et al. [59], the recruitment of p62 and NDP52 to Shigella is interdependent. These adaptors subsequently recruit specific autophagic machinery components, such as the LC3/Atg8 family proteins, triggering the autophagic cascade and autophagosome formation (initiation, elongation and closure).