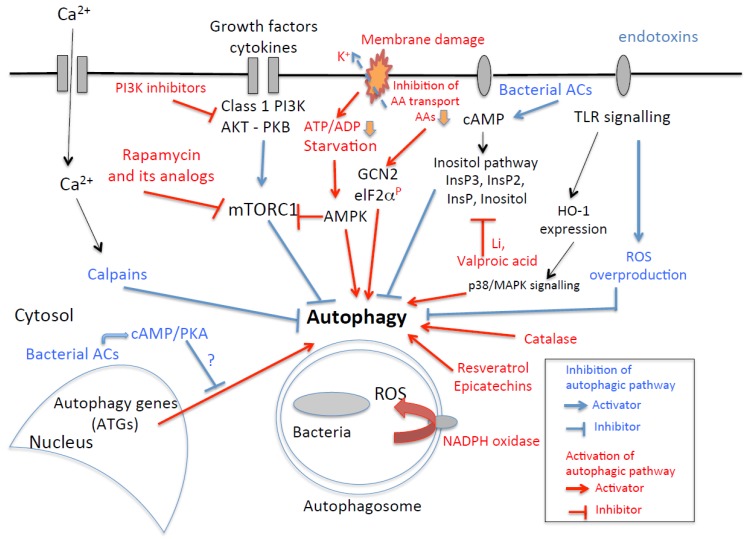
Figure 3.
A simplified overview (a modified schema from [2]) of some molecular events (and potential drug targets) involved in bacterial autophagy. For example, the increase in intracellular calcium concentration leads to thea activation of calpains, which inhibit autophagy. Inhibitors of calcium or calpain could therefore interfere with the autophagic pathway. Inflammatory mediators, such as cytokines, trigger PI3K activation, leading to a decrease in autophage through effects on mTORC1. PI3K inhibitors or rapamycin treatment to inhibit mTORC1 enhances autophagy. Glucose starvation, which can be induced by stress, activates AMPK, leading to the inhibition of mTORC1 and the stimulation of autophagy. Membrane damage by pore-forming toxins induces starvation responses [120]. Loss of cellular potassium from perforated cells leads to the failure of nutrient transport and transient drop of ATP, thus activating cellular nutrient and energy sensors GCN2 and AMPK, subsequent phosphorylation of ElF2α and deactivation of mTORC1. Bacterial toxins, such as those with adenylate cyclase activity (ACs), enhance cAMP production, leading to an activation of the inositol pathway. InsP3 inhibits autophagy, and drugs such as lithium (Li) and valproic acid block the inositol cycle, restoring autophagy. The overproduction of cAMP activates the PKA pathway, potentially impairing the localization of Atg1 and Atg13 to the phagophore assembly site, resulting in defective autophagy. Motifs from bacteria or endotoxins are recognized by TLRs, which activate numerous pathways. For example, those triggering the HO-1 pathway can activate autophagy by activating mitochondrial ROS and p38 MAPK. However, endotoxins can induce oxygen stress and ROS overproduction, via the TLRs, either enhancing or inhibiting autophagy. Antioxidants, such as catalase and resveratrol, strongly enhance or restore the autophagic pathway.