Table 1. The pharmacology of the endocannabinoid system–selected compounds.
| Compound | Mechanism of action | Effect in pain |
|---|---|---|
THC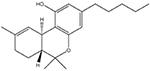
|
Primarily activation of CB receptors, although has off-target actions (e.g. Barann et al., 2002) |
Inflammatory: + Neuropathic: + |
AM1241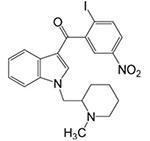
|
CB2-receptor selective ligand; acts as a “protean” agonist in vitro (Yao et al., 2006) and CB2 agonist in vivo |
Inflammatory: + Neuropathic: + |
GW405833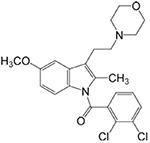
|
CB2-receptor selective ligand (efficacy dependent upon assay used, see Yao et al., 2008) |
Inflammatory: + Neuropathic: + |
LY2318912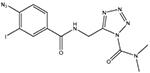
|
Blocks the accumulation and metabolism of AEA (Moore et al., 2005). Acts primarily as a potent FAAH inhibitor, but with many off-target actions (Alexander and Cravatt, 2006) |
Inflammatory: + |
URB597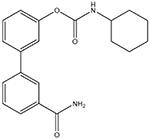
|
Selective FAAH inhibitor (Kathuria et al., 2003). Some off-target actions have been reported, but their importance is unclear |
Inflammatory: + Visceral: + Neuropathic: +/− |
ibu-am5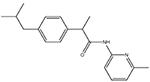
|
Dual COX- and FAAH-inhibitory compound (Holt et al., 2007). |
Visceral: + |
| N-arachidonoylserotonin |
Dual TRPV1 antagonist/FAAH-inhibitory compound (Maione et al., 2007). |
Inflammatory: + Neuropathic: + |
Pravadoline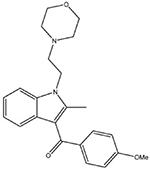
|
Dual CB agonist/COX-inhibitory compound (D’Ambra et al., 1992). |
Inflammatory: + Visceral: + |
The compounds shown in this table have been selected since they are those principally discussed in this review.“+” indicates efficacy in a preclinical model of this pain type (for details, see text). For a more complete list, see Fowler (2008).
