Abstract
Background and Aims The occurrence of Arabidopsis thaliana semi-dwarf accessions carrying inactive alleles at the gibberellin (GA) biosynthesis GA5 locus has raised the question whether there are pleiotropic effects on other traits at the root level, such as rooting depth. In addition, it is unknown whether semi-dwarfism in arabidopsis confers a growth advantage under water-limiting conditions compared with wild-type plants. The aim of this research was therefore to investigate whether semi-dwarfism has a pleiotropic effect in the root system and also whether semi-dwarfs might be more tolerant of water-limiting conditions.
Methods The root systems of different arabidopsis semi-dwarfs and GA biosynthesis mutants were phenotyped in vitro using the GROWSCREEN-ROOT image-based software. Semi-dwarfs were phenotyped together with tall, near-related accessions. In addition, root phenotypes were investigated in soil-filled rhizotrons. Rosette growth trajectories were analysed with the GROWSCREEN-FLUORO setup based on non-invasive imaging.
Key Results Mutations in the early steps of the GA biosynthesis pathway led to a reduction in shoot as well as root size. Depending on the genetic background, mutations at the GA5 locus yielded phenotypes characterized by decreased root length in comparison with related wild-type ones. The semi-dwarf accession Pak-3 showed the deepest root system both in vitro and in soil cultivation experiments; this comparatively deep root system, however, was independent of the ga5 loss-of-function allele, as shown by co-segregation analysis. When the accessions were grown under water-limiting conditions, semi-dwarf accessions with high growth rates were identified.
Conclusions The observed diversity in root system growth and architecture occurs independently of semi-dwarf phenotypes, and is probably linked to a genetic background effect. The results show that there are no clear advantages of semi-dwarfism at low water availability in arabidopsis.
Keywords: Arabidopsis thaliana, semi-dwarfs, phenotyping, gibberellin biosynthesis, GA5, root system, low water availability, drought tolerance, GROWSCREEN
INTRODUCTION
Bioactive gibberellins (GA) are plant growth regulators responsible for the expression of several plant traits (Sun, 2008). Their biosynthesis and signalling pathways are well understood (Yamaguchi, 2008; Hedden and Thomas, 2012). Gibberellin-related mutations have played an important role in crop breeding because mutations in the GA signalling and biosynthesis pathways induce semi-dwarfism. These mutants have contributed to yield increases, particularly in wheat and rice, leading to the green revolution (Hedden, 2003; Salamini, 2003), especially by conferring lodging resistance. Semi-dwarf mutants that were selected by plant breeders had no negative pleiotropic effects (Sasaki et al., 2002; Spielmeyer et al., 2002; Jia et al., 2011) on yield-related traits and may have positively acting pleiotropic effects, such as those on the root system. However, effects on root growth are more difficult to measure systematically and quantitatively and this aspect has received little attention.
Root growth is regulated by plant hormones, and the role of auxins has been studied in detail (Overvoorde et al., 2010). Auxin gradients control the length of the primary root, the number of lateral root primordia and the response to gravity (Overvoorde et al., 2010). A recent review showed that GA could also regulate root elongation and thickening in the elongation zone of roots (Tanimoto, 2012). In that paper the author collected examples showing the sensitivity of shoots, hypocotyls and roots to GA and GA inhibitors and provides arguments supporting the view that roots have a higher GA sensitivity compared with shoots. In Arabidopsis thaliana, application of GA to the shoot increases the elongation of primary roots (Bidadi et al., 2010). Interestingly, this study records that application of GA inhibitors to the shoot also increases root elongation. This is probably due to negative feedback of GA levels on de novo synthesis (reviewed in Yamaguchi, 2008). Studies in Populus showed that GA-deficient mutants (with increased expression of GA2ox1) and GA-insensitive mutants (overexpression of RGL1) increase lateral root density and elongation (Elias et al., 2012). Crosstalk with the auxin hormone pathway may occur (Gou et al., 2010).
Several naturally occurring arabidopsis accessions carrying non-functional ga5 alleles were identified (Barboza et al., 2013; Luo et al., 2015). The GA5 gene encodes GA 20-oxidase1 (a GA biosynthesis enzyme), causing semi-dwarfism when mutated (Koornneef and van der Veen, 1980; Xu et al., 1995). The occurrence of semi-dwarfism in nature indicates that positive selection in certain populations may confer an advantage under specific environmental conditions. Recent studies suggest that semi-dwarfism is involved in adaptation to high altitudes (Luo et al., 2015). However, this may not be the only reason why semi-dwarfism occurs, because this phenotype is present in many coastal (low-altitude) regions of the Netherlands and Scandinavia (Barboza et al., 2013). GA5 is the functional orthologue of the SD1 rice gene and of the Sdw/Denso barley gene. Mutations in both genes lead to semi-dwarfism in modern rice and barley cultivars. Modifications of root growth can have an impact on the growth performance of plants under stress conditions and genetic variation in the hormonal pathways may affect root traits (Ghanem et al., 2011). There is experimental evidence that modern semi-dwarf barley genotypes have a longer root system compared with non-dwarf ones and quantitative trait locus (QTL) alleles controlling plant height co-localize with QTLs for root system size (Chloupek et al., 2006). Furthermore, dwarf alleles play a role in nitrogen acquisition under low nutrient supply, since it has been found that the sdw1 dwarf allele in barley has a smaller root mass under reduced nutrient supply compared with long-stem varieties (Karley et al., 2011). A study by Vartanian et al. (1994) using arabidopsis showed that the ga5 mutant has a drought-stress-adapted root system that might be more effective than the corresponding wild type in water acquisition. This was measured in water-withholding pot experiments studying the number of short roots present along lateral root axes (Vartanian et al., 1994). It remains to be established under which water-limiting scenario this trait can be beneficial. Moreover, recent studies showed the important role of the DEEPER ROOTING 1 (DRO1) mutation in rice, which increased rooting depth (>2-fold in Dro1-NIL compared with its genetic background, IR64) and conferred drought tolerance (Uga et al., 2013). Deeper roots may be advantageous in water capture from the subsoil in dry environments. To our knowledge, there are no studies describing the root system of natural arabidopsis semi-dwarfs or growth performance under water-limiting conditions.
The aim of our study was to (1) characterize the shoot and root system of GA biosynthesis mutants and selected arabidopsis accessions carrying functional and mutated alleles of the GA5 gene, and (2) to evaluate their response to reduced water availability. If mutations at the GA5 locus have positive pleiotropic effects on root growth, this could help explain their selective advantage in specific environments, with particular reference to reduced water availability.
MATERIALS AND METHODS
Plant material
The selection of genotypes of Arabidopsis thaliana used in these experiments (Table 1) is based on previous studies (Barboza et al., 2013). For the QTL mapping experiment, the Ler × Kas-2 recombinant inbred line (RIL) population was used (El-Lithy et al., 2005). In previous experiments, all plants were propagated by single-seed descent together in a greenhouse at the Max Planck Institute for Plant Breeding Research, Germany.
Table 1.
List of genotypes included in the research
| Genotype | Type | Height phenotype | Reference/collector |
|---|---|---|---|
| Kas-2 | Accession | Semi-dwarf | Sharma |
| Kas-0 | Accession | Wild type | Sharma |
| Kl-2 | Accession | Semi-dwarf | Hülsbruch |
| Je-0 | Accession | Wild type | Platt et al., 2010 |
| Pak-3 | Accession | Semi-dwarf | J. Mirza |
| Pak-1 | Accession | Wild type | J. Mirza |
| OW-0 | Accession | Semi-dwarf | M. Koornneef |
| OW-1 | Accession | Wild type | M. Koornneef |
| Sparta | Accession | Semi-dwarf | M. Nordborg |
| T620 | Accession | Wild type | Platt et al., 2010 |
| Dja-1 | Accession | Semi-dwarf | O. Loudet |
| Neo-3 | Accession | Wild type | O. Loudet |
| Col | Accession | Wild type | N907a |
| Ler | Accession | Wild type | NW20a |
| ga20ox2-1 | Mutant (Col) | Wild type | Rieu et al., 2008 |
| ga20ox1-3 | Mutant (Col) | Semi-dwarf | Rieu et al., 2008 |
| ga20ox1 ga20ox2 | Double mutant (Col) | Semi-dwarf | Rieu et al., 2008 |
| ga1-13 | Mutant (Col) | Dwarf | Alonso et al., 2003 |
| ga1-3 6xbxcol | Mutant (Col) | Dwarf | Mitchum et al., 2006 |
| ga3ox1-3 | Mutant (Col) | Semi-dwarf | Mitchum et al., 2006 |
| ga3ox1-3 ga3ox2-1 | Double mutant (Col) | Dwarf | Mitchum et al., 2006 |
| gai | Mutant (Ler) | Semi-dwarf | Koornneef et al., 1985 |
| ga4 | Mutant (Ler) | Semi-dwarf | Koornneef and van der Veen, 1980 |
| ga5 | Mutant (Ler) | Semi-dwarf | Koornneef and van der Veen, 1980 |
aNottingham Arabidopsis Stock Center (NASC) identification number.
In vitro root experiments
In a first approach, to understand the role of different mutations in the GA biosynthesis and signalling pathway, different mutants (Table 1) were phenotyped. All experiments were conducted in a complete randomized design. In a second approach, to characterize the phenotype of the semi-dwarf accessions an experiment was conducted with 12 natural accessions (Table 1) together with the ga5 mutant and its parental wild type, Ler. Ler and the ga5 mutant were included in all experiments. Experiments were performed from 11 June 2012 to 13 July 2012. Root systems were phenotyped in vitro using the GROWSCREEN-ROOT system with shoots growing outside the agar plate and roots growing within the agar medium (Nagel et al., 2009). The culture medium contained one-third Hoagland solution and 1 % agar as described in previous studies (Nagel et al., 2009). Plants were grown on 120 × 120 × 17-mm plates (Greiner) filled with ∼ 166 mL of medium. Seeds were sterilized with 70 % ethanol (3 min) followed by 0·5 % NaOCl (10 min), and were then rinsed three times with sterile Milli-Q H2O. After sowing, seeds were incubated at 4 °C in the dark for 5 d. Subsequently, the plates were incubated vertically in a growth cabinet (Bioline VB 1100 Vario; Vötsch Industrietechnik, Germany) set to an 8/16-h light/dark period, 22 °C day/18 °C night temperature and 60 % air humidity. Each plate contained four plants of the same genotype and there were three replications (n = 12). Plants were phenotyped and shoots were harvested and immediately weighed to obtain the fresh weight at day 18 after germination (during the morning, 0900–1200 h). Dry weight of shoots was determined after samples had been oven-dried at 70 °C for ∼48 h or until constant weight was reached. The evaluated root and shoot traits are summarized in Table 2.
Table 2.
Traits evaluated in the experiments conducted in vitro
| Abbreviation | Trait | Quantification method |
|---|---|---|
| LLR | Length lateral roots | Nagel et al., 2009 |
| LPR | Length primary root | Nagel et al., 2009 |
| RSD | Root system depth | Nagel et al., 2012 |
| RSW | Root system width | Nagel et al., 2012 |
| TRL | Total root length | Nagel et al., 2009 |
| PLA | Projected leaf area | Walter et al., 2007 |
| SDW | Shoot dry weight | Gravimetric |
| SFW | Shoot fresh weight | Gravimetric |
| RSR | Root to shoot ratio | RSD/SFW |
| LDMC | Leaf dry matter content | SDW/SFW |
| SLA | Specific leaf area | PLA/SDW |
| PLT | Proxy leaf thickness | SLA × LDMC |
Additional experiments were conducted to measure root system depth (RSD) in the Ler × Kas-2 RIL population (El-Lithy et al., 2005) and in F2 lines derived from the cross Ler × Pak-3. Plants were grown in half-strength Murashige and Skoog medium (Duchefa), pH 5·8, 0·8 % agar (Plant Agar; Duchefa), 100 µL L−1 Plant Preservative Mixture (PPM). In all experiments, seeds were stratified for 5 d at 4 °C in the dark. The plates used were the same as those described above. In this case plants were grown inside the plate, with six plants per plate. Experiments were conducted from 12 April 2013 to 3 May 2013. After stratification, seeds were transferred to a growth chamber (KL 01 U KTG MC; Van den Berg Klimaattechniek, The Netherlands) at 25/20 °C (day/night). Plants were phenotyped with a digital calliper (Series 500, 0–200 mm; Mitutoyo, Japan) at day 21 after germination (during the morning, 0900–1200 h).
The mutants ga1-13, ga1-3 6xbxcol and ga3ox1-3 ga3ox2-1 were stratified in 100 µm GA4+7 solution (Duchefa) to ensure germination under the same temperature conditions as those described above. The GA stock solution was at a concentration of 25 mm (GA dissolved in a few drops of 1 m KOH).
Rhizotron experiments
An experiment was conducted with six arabidopsis accessions, the ga5 mutant and Ler. Plants were grown in rhizotrons (60 × 30 × 2 cm) filled with peat soil (Graberde; Plantaflor Humus, Vechta, Germany; containing N, ∼120 mgL–1; P2O5, ∼20 mgL–1; K2O, ∼170 mgL–1), as described in previous studies (Nagel et al., 2012). Seeds were sown directly onto the surface of the soil-filled rhizotrons and stratified for 4 d at 4 °C (18 February 2013). Afterwards, plants were grown at 23 ± 10 °C, 53 ± 33 % relative humidity in the PhyTec greenhouse of the Institute of Plant Sciences (IBG-2) at Forschungszentrum Jülich, Jülich, Germany. One plant per rhizotron was grown. Each genotype was tested with six repetitions. To account for microclimate variation, a randomized spatial layout was used in these experiments. The plants were supplied with tap water (electrical conductivity ∼440 mS cm–1) twice per week. The amount of irrigation water was adjusted taking into account plant age (60 mL until a plant age of 2 months, 100 mL in the third month after sowing). Root system depth was quantified by image processing using ImageJ (Schneider et al., 2012). Images were acquired once a week, during the morning (0900–1200 h, starting 7 March 2013 and finishing 10 May 2013) using a single-lens reflex camera (Canon EOS 40D, Canon EF 24–70 mm zoom lens).
Above-ground phenotyping experiments
Water-withholding experiments were conducted using pot-grown plants measured with the GROWSCREEN-FLUORO setup (Jansen et al., 2009) built into a climate-controlled growth chamber (SCREEN chamber) and integrated into an automated plant evaluation routine. For plant cultivation, single seeds were sown in 576-well trays filled with soil (Pikiererde; Balster Einheitserdewerk, Fröndenberg, Germany). Stratification was conducted for 4 d at 4 °C. The trays were then moved to the growth chamber (8/16-h light/dark period, 22 °C day/18 °C night temperature, air humidity 50 %). After cotyledon unfolding, seedlings were transplanted to pots (7 × 7 × 8 cm) filled with peat–sand–pumice substrate (SoMi 513 Dachstauden; Hawita, Vechta, Germany). Pots were randomized and arranged in trays (40 pots per tray). Pots of both control (well-watered) and treated (water-withholding) groups were water-saturated upon transplanting, reaching a volumetric soil water content (cm3/cm3) of ∼60 %. During seedling establishment after transplantation, the pots were progressively dried until reaching 30 % volumetric soil water content. They were maintained at this moisture level until the start of the water-withholding treatment based on gravimetric measurements and adjustment of the irrigation schedule (week 4). Pots of the control group were kept at this moisture level for the rest of the experiment, whereas the treated group was not irrigated until volumetric soil water content reached ≤6 %. At this soil moisture level the plants stopped growing, as determined by analysis of the projected rosette area (Jansen et al., 2009). Re-watering was then done (week 6) up to 30 % volumetric soil water content of the control group and plants were allowed to recover for 1 week. Based on the analysis of the water retention curves conducted in 2013 at the University of Kiel, Institute of Plant Nutrition and Soil Science, Germany, the corresponding estimated water potential of the soil used for this experiment was 0 MPa at 60 % cm3/cm3, –0·03 MPa at 30 % cm3/cm3 and less than –1·5 MPa, i.e. below the permanent wilting point, at 6 % cm3/cm3. These water potential values were estimated using eight-point curves that were fitted with the van Genuchten equation (van Genuchten 1980).
The first experiment was conducted with a group of GA biosynthesis and signalling mutants (conducted from 26 July 2012 to 14 September 2012). In the second experiment, natural accessions together with ga5 and Ler were studied (conducted from 7 September 2012 to 26 October 2012). Each genotype had 10–20 repetitions (depending on the experimental setup). A complete randomized design was used. Gravimetric monitoring of tray water content, water supply and GROWSCREEN FLUORO data acquisition and shoot trait evaluation were conducted as described in previous studies (Jansen et al., 2009). Projected leaf area measurements took place five times per week; upon each measurement the positions of the trays in the growth room were changed in order to compensate for position effects. On the last day of evaluation the shoots were harvested and immediately weighed to obtain the fresh weight (during the morning, 0900–1200 h). Dry weights of shoots were determined as described above. The mutants ga1-13, ga1-3 6xbxcol and ga3ox1-3 ga3ox2-1 were germinated as described for the in vitro root experiments.
Data analysis
Descriptive statistics and correlations were calculated and ANOVA was performed with R software (R Core Team, 2014). Post hoc Tukey tests were conducted using the R package Agricolae (de Mendibur, 2014). Principal components analysis (PCA) was done with the package FactoMineR (Lê et al., 2008). To estimate the area under the curve (AUC) for the projected leaf areas, the R package MESS (Ekstrom, 2014) was used. In order to estimate confidence intervals for the AUC ratios, 1000-fold bootstrapping of the AUC estimation was performed, using the R package boot (Canty and Riple, 2014). The R/qtl package was used to map QTL (Broman et al., 2003; Arends et al., 2010). The function mqmscan was used, setting all markers as cofactors, with further processing by backward elimination. To quantify the explained variance, QTLs above the 5 % significance threshold (using 1000 permutations) were selected and fitted into a multiple QTL model using the function fitqtl based on the Haley–Knott regression method.
RESULTS
Gibberellin biosynthesis mutants exhibit reduced shoot and root development in vitro
To evaluate the role of mutations in the GA biosynthesis pathway in the modulation of root growth, different GA-deficient mutants were phenotyped in vitro using the agar-based GROWSCREEN-ROOT assay system (Nagel et al., 2009). Semi-dwarf mutants in the GA20ox1 (GA5) locus were included in the two backgrounds, Ler (ga5) and Col (ga20ox1-3, ga20ox1 ga20ox2). Additional mutants with semi-dwarf (ga3ox1-3) and extreme dwarf (ga1-13, ga1-3 6xbxcol) phenotypes were included. All mutants exhibited decreased shoot fresh weight (SFW) at day 18, except ga20ox1-3 and ga3ox1-3 ga3ox2-1, which had a phenotype comparable to that of their backgrounds (Fig. 1A). None of the mutants showed a significant decrease in RSD, or even any change in RSD, except ga3ox1-3 (Fig. 1B). The ga5 mutant mainly showed a significant decrease in SFW (Fig. 1A), projected leaf area (Supplementary Data Fig. S1) and proxy leaf thickness (calculated by multiplying specific leaf area by leaf dry matter content; Table 2, Supplementary Data Fig. S1). The results indicated that the GA 20-oxidase 1 (ga5) mutation leads to a more pronounced growth reduction in terms of SFW on the Ler background compared with the Col background because differences between ga5 and Ler were greater (29·7 % reduction compared with wild type) than those observed between ga20ox1-3 and Col (15·0 % reduction compared with wild type). These findings were supported by PCA using all evaluated traits, in which ga20ox1-3, ga20ox2-1 and ga20ox1 ga20ox2 tended to cluster near their background accession Col while the ga5 mutant was further from its wild-type genetic background, Ler (Supplementary Data Fig. S2).
Fig. 1.
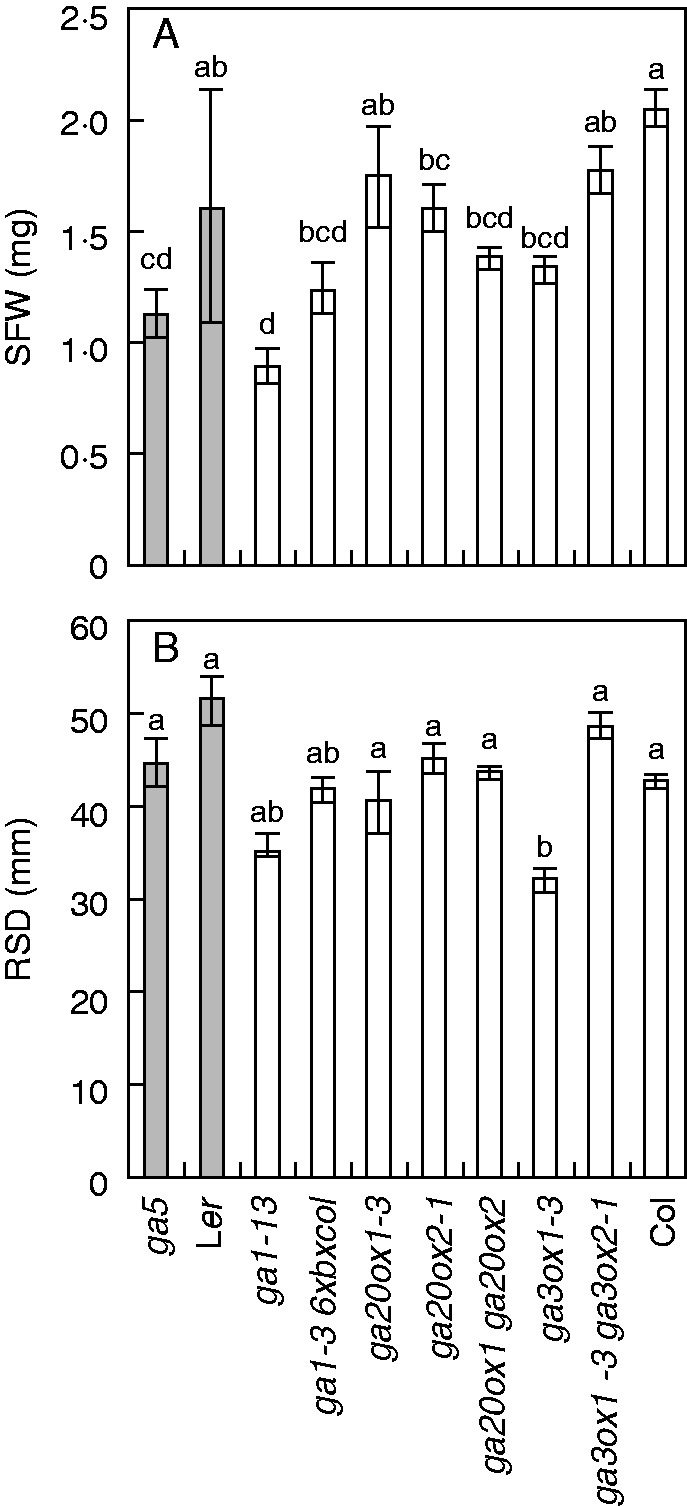
Phenotypes of arabidopsis GA biosynthesis mutants. Means ( ± s.e., n = 4–12) of (A) shoot fresh weight (SFW) and (B) root system depth (RSD) in different GA biosynthesis mutants and their corresponding wild-type controls, grown in vitro (1 % agar with 1/3 Hoagland solution, evaluated 18 d after germination). All mutants are on the Col background except ga5, which has the Ler background. Different shadings indicate near-isogenic comparisons. The letters above each column indicate the results of a post hoc Tukey’s HSD test; means with different letters are significantly different (P < 0·05).
Root phenotype of semi-dwarf accessions depends on the genetic background
We characterized the root system architecture of several semi-dwarf accessions allelic to ga5 (Barboza et al., 2013) and related non-dwarf genotypes. Variation among accessions carrying active and inactive ga5 alleles was observed, suggesting no consistent effect of semi-dwarfism for the evaluated traits. The semi-dwarf accession Kas-2 had the highest SFW value, while the semi-dwarf accession Kl-2 had the lowest (Fig. 2A). The semi-dwarf Pak-3 showed the longest RSD and the non-dwarf Kas-0 the shortest RSD (Fig. 2B). The PCA analysis showed no clustering of semi-dwarf accessions, suggesting that semi-dwarfism does not contribute per se to variation in the evaluated traits (Supplementary Data Fig. S3A). However, this PCA analysis indicated that Pak-3 is an outlier, mainly because of its deeper root system and the length of its primary root (Supplementary Data Fig. S3B). It was possible to test the semi-dwarf effect among the traits because we included a balanced number of accessions carrying active and inactive ga5 alleles. Remarkably, no semi-dwarf effect was significant. Only root system width, representing the maximum horizontal distribution of a root system, was significantly different between dwarf and wild-type genotypes (P < 0·001; mean value for semi-dwarfs 9·0 ± 0·4 mm versus 12·8 ± 0·8 mm for wild type). Neo-3 showed an increased root system width compared with the other accessions (mean ± s.e. under control conditions 23·8 ± 1·6 mm versus 7·3 ± 1·0 mm for the semi-dwarf Dja-1, a genetically related accession). However, when the non-dwarf Neo-3 was excluded from the analysis the overall effect was not statistically significant (P > 0·05). These observations lead to the conclusion that shoot and root modifications depend on the genetic background, which might interact to some extent with the ga5 genotype. Apparently, variation at additional loci contributes to genotype differences for the evaluated traits.
Fig. 2.
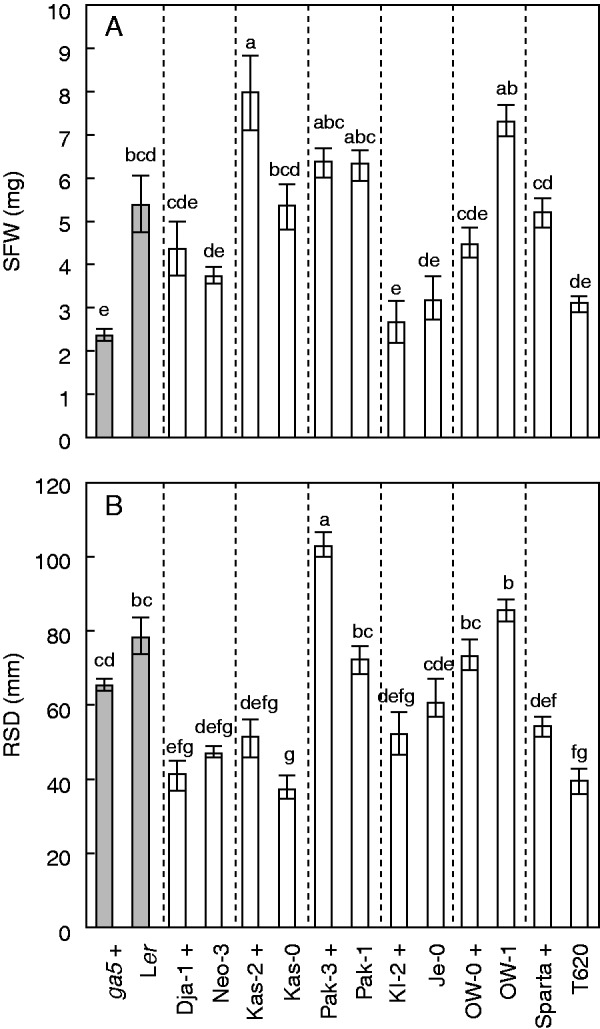
Contrasting phenotypes in the semi-dwarf accessions Pak-3 and Kas-2. (A) Shoot fresh weight (SFW) and (B) root system depth (RSD) (mean ± s.e., n = 6–12) in 13 arabidopsis accessions and the ga5 mutant (Ler background) grown in vitro (1 % agar with 1/3 Hoagland solution, evaluated 18 d after germination). Semi-dwarf phenotypes are indicated by ‘+’ after the accession name. The letters above each panel indicate the results of post hoc Tukey’s HSD test; means with different letters are significantly different (P < 0·05). Vertical dotted lines separate near-related accessions comparisons.
We used QTL analysis as an independent approach to test the possible role of ga5 in controlling shoot biomass and RSD in vitro using the Ler × Kas-2 population (El-Lithy et al., 2005). The advantage of using this population is that semi-dwarfism due to the Kas-2 loss-of-function allele at the GA5 locus is segregating, which allows us to test the effect of ga5 on RSD. Transgressive segregation was observed for SFW and RSD (Supplementary Data Fig. S4). When tested separately for each trait, ga5 reduced both RSD and SFW, but this effect was relatively small and the log of the odds (LOD) score was not significant (Supplementary Data Figs S5 and S6). A locus at or near ERECTA (chromosome 2) and a locus located on chromosome 5 (SNP304) controlled mainly SFW (LOD scores 5·0 and 4·1) and the amount of variance explained was 14·3 and 10·6 %, respectively (Supplementary Data Fig. S6). The ERECTA locus might play a minor role in RSD, together with a QTL on chromosome 4 (M4-3), but no QTL position was detected for RSD in the vicinity of the ga5 locus.
Pak-3, a semi-dwarf accession, showed the greatest rooting depth among all those tested (Fig. 2B). All observed phenotypes in the evaluated natural semi-dwarf accessions led to the conclusion that semi-dwarfism does not affect this trait. Nevertheless, indications that barley semi-dwarf accessions show a higher root length (Chloupek et al., 2006) raised the question of whether or not the long Pak-3 RSD is dependent on the ga5 mutation. To test this hypothesis, a co-segregation analysis was conducted by phenotyping RSD and plant height in F2 populations derived from the hybrids ga5 × Pak-3 and Ler × Pak-3. The goal of this experiment was to quantify whether RSD and shoot height variation (due to ga5 genotype) were correlated. Clear differences for both plant height phenotype and RSD were observed. However, these traits segregated independently of each other (Supplementary Data Fig. S7). We conclude that ga5 does not play a role modifying RSD in the Pak-3 accession.
To further characterize the evaluated semi-dwarf accessions, a selected group of genotypes (ga5, Ler, OW-0, OW-1, Dja-1, Neo-3, Pak-3, Pak-1) was phenotyped in rhizotrons using soil as substrate. As observed in the agar plate experiments, the Pak-3 accession had the greatest RSD 4 weeks after sowing (Fig. 3). In contrast, another Central Asian semi-dwarf accession, Dja-1, presented the smallest rooting depth, which did not differ significantly from that of the related wild type, Neo-3. The semi-dwarf mutant ga5 showed a smaller RSD compared with its wild type, Ler. A final evaluation was performed 2 weeks after flowering to phenotype these genotypes for plant height. As previously reported (Barboza et al., 2013), the ga5 mutation only had a significant effect on average plant height (P < 0·0001), while differences in the root system at this stage were apparently independent of this locus (Fig. 4).
Fig. 3.
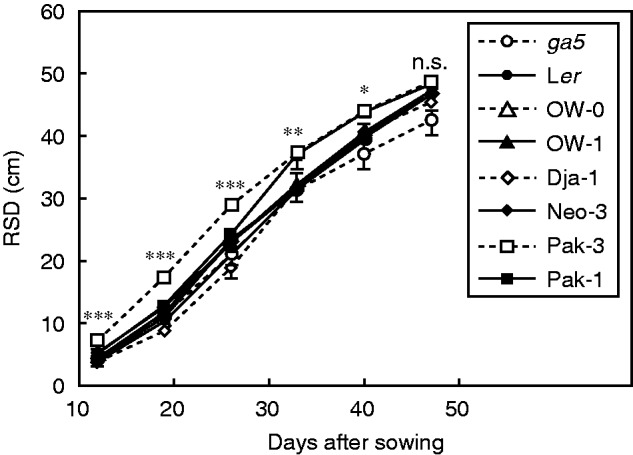
Root system depth (RSD) of soil-grown accessions. Development of RSD across time in seven arabidopsis accessions and the ga5 mutant (Ler background) grown in soil-filled rhizotrons. Data are mean ± s.e. (n = 3–6). Asterisks show P values from ANOVA to test for significant differences among genotypes: ***≤ 0·001, **≤ 0·01, *≤ 0·05, n.s. not significant (P > 0·05). Dashed lines represent genotypes with semi-dwarf phenotype. Similar marker styles indicate near-related accessions.
Fig. 4.
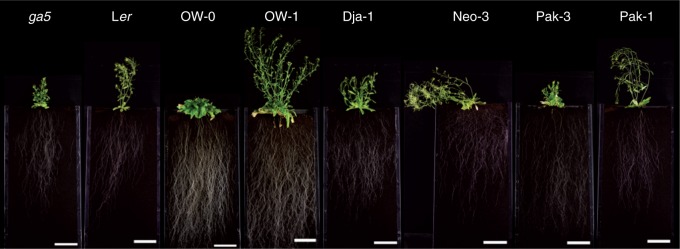
Arabidopsis accessions and the ga5 mutant (Ler background) grown in soil-filled rhizotrons. Photographs of representative individuals were taken 2 weeks after flowering. Near-related accessions are next to each other. Scale bar = 10 cm.
Responses to reduced water availability
The presence of semi-dwarfs in nature indicates that this trait might confer a selective advantage under specific conditions, in particular in environments characterized by the occurrence of abiotic stresses. Previous studies suggested that ga5 might be more drought-tolerant due to modifications of root system architecture (Vartanian et al., 1994). We quantified the performance of GA biosynthesis mutants and natural semi-dwarf accessions under reduced water availability. To create water stress in agar plates, an experiment using osmotic stress was conducted by applying sorbitol (100 mm). The percentage of growth (relative to control) for all evaluated traits was compared. No strong differences were observed when comparing growth percentage for the different evaluated GA mutants (Supplementary Data Table S1). Only leaf dry matter content (LDMC) was different when comparing ga5 with Ler (164·5 ± 21·0 versus 61·2 ± 30·4 %) and mutant ga20ox1-3 with Col-0 (151·4 ± 22·7 versus 114·8 ± 6·4 %). Also, the double mutant ga20ox1 ga20ox2 showed a higher LDMC growth percentage compared with its background accession Col-0 (150·1 ± 57·8 versus 114·8 ± 6·4 %). Different accessions were tested under the same conditions. This experiment did not show significant effects of semi-dwarfs compared with wild-type accessions (Supplementary Data Fig. S8). The semi-dwarf Dja-1 showed the best performance (112 % growth relative to control), indicating this accession was not affected by the sorbitol treatment.
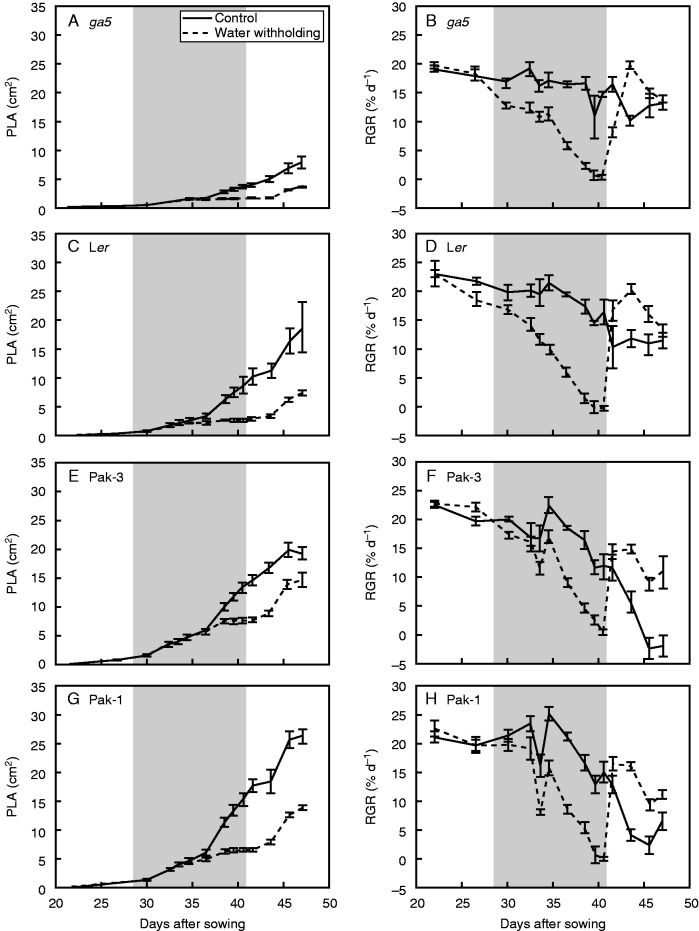
To test a different water limitation scenario, water-withholding experiments using pots filled with soil were conducted. For this experiment the mutants ga4 (encoding the GA biosynthesis enzyme GA3ox1, causing semi-dwarfism when mutated), gai (GA signalling mutant causing dwarfism when mutated) and aba1-3 [abscisic acid (ABA)-deficient mutant; (Koornneef et al., 1982)] were included (all on the Ler background). The mutant aba1-3 showed the lowest performance for SFW under the water-limitation treatment (Fig. 5A). Among the induced mutants, ga5 showed a higher growth percentage compared with its background accession Ler for the trait SFW (Fig. 5A). However, the allelic ga20ox1-3 mutant did not show differences from its background accession Col-0 (Fig. 5A). The double mutant ga20ox1 ga20ox2 showed the lowest performance among the Col background accessions. Among the accessions, Pak-3 showed the highest growth percentage for SFW (Fig. 5B). The ga5 mutant showed a better performance in SFW (in soil, water withholding) than Ler (Fig. 5B). The use of GROWSCREEN-FLUORO (Jansen et al., 2009) allows the estimation of growth in time during and after drought using projected leaf area (PLA; Fig. 6). The output using PLA across time resembled the results obtained using SFW (Fig. 5A), in which the mutant aba1-3 showed the lowest growth rate during both the water-withholding and the re-watering phase (Fig. 6A). Wide variation among the remaining genotypes (especially for Ler) suggests a similar response, but it should be noted that the semi-dwarfs gai and ga4 showed a moderate response (Fig. 6A). When this effect was evaluated in different accessions, the Pak-3 accession presented a high growth rate during water-limiting conditions, together with the semi-dwarf Kas-2 and the non-dwarf Je-0 (Fig. 6B). Besides, Pak-3 had the highest growth rate during the recovery phase (Fig. 6B). The F1 populations of the crosses ga5 × Pak-3 and ga5 × OW-0 showed no differences under water-limiting conditions compared with crosses of mutants with wild-type Ler (data not shown). In this experiment (independently conducted), ga5 tended to have a higher growth rate than Ler (higher in the first experiment). Of note, ga5 had a smaller PLA over time compared with Ler (Fig. 7A, C), but despite this the relative growth rates (RGRs) of the two genotypes were similar (Fig. 7B, D). The differences between control and water-withholding treatments for the trait PLA tended be lower in ga5 than in Ler (Fig. 7A, C). The same was observed when comparing the semi-dwarf Pak-3 with the wild-type Pak-1 (Fig. 7E–H).
Fig. 5.
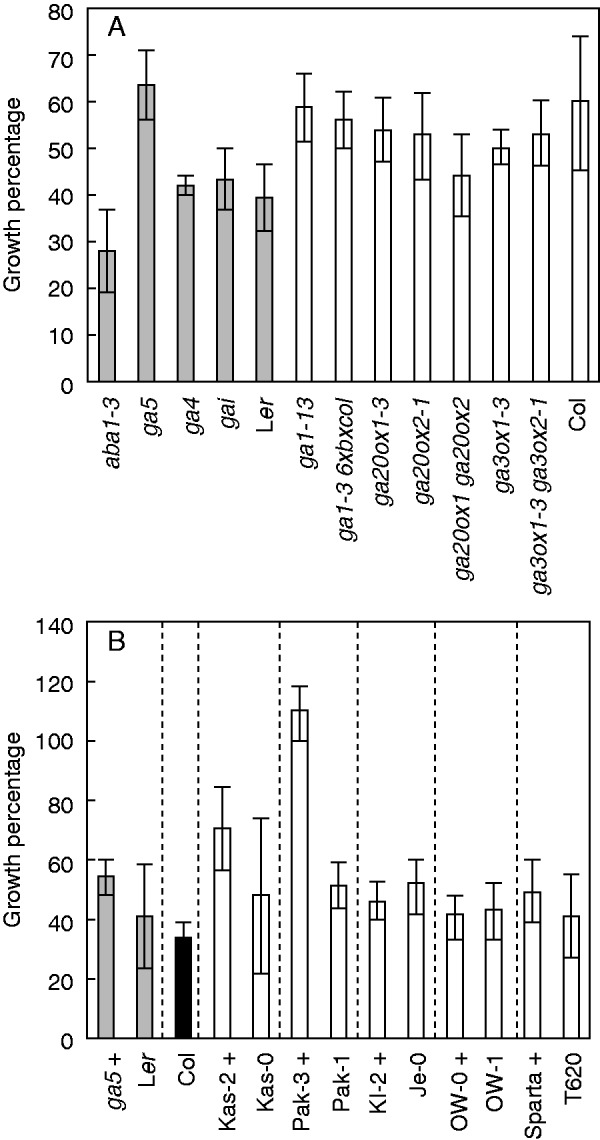
Water withholding and response (growth percentage under water-limiting conditions relative to control) among arabidopsis GA biosynthesis and signalling mutants and natural accessions. (A) Growth percentages for shoot fresh weight (SFW) representing variation among mutants and (B) natural accessions grown in soil on pots after withholding of water followed by 1 week of recovery after re-watering. Shoots were harvested at week 7. In (A), different shadings indicate near-isogenic comparisons. Growth percentages were obtained using mean values (water withholding/control) ± uncertainties (n = 4–14 for water withholding, n = 4–12 for control). Vertical dotted lines in (B) separate near-related accessions. Semi-dwarf phenotypes are indicated by ‘+’ after the accession name.
Fig. 6.
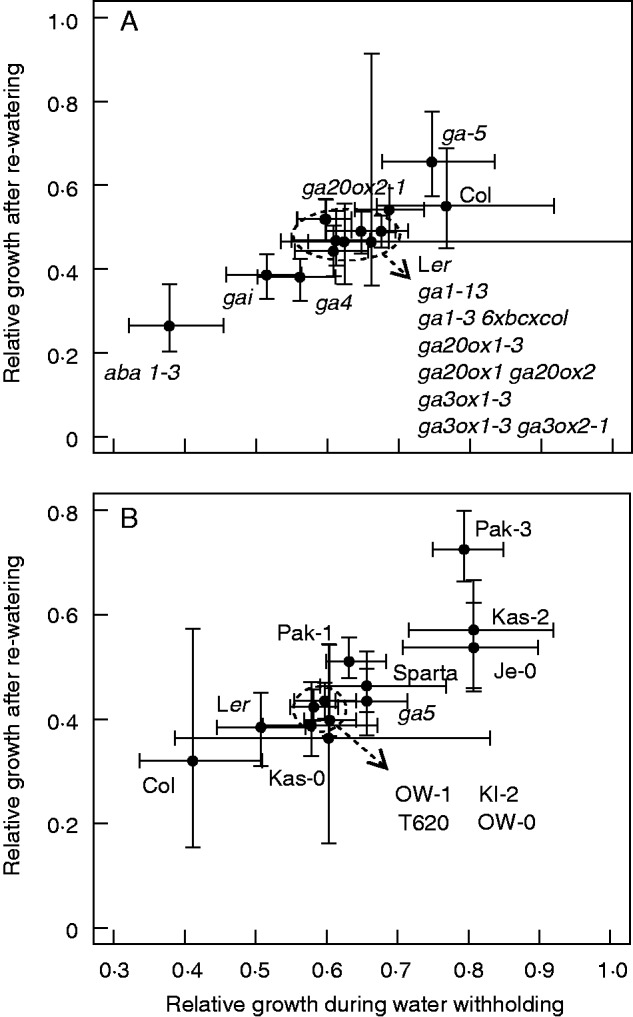
Water-withholding responses among arabidopsis (A) GA biosynthesis and signalling mutants, and (B) natural accessions grown in soil. Ratios were obtained by dividing the water-withholding value by the control value for the area under the curve for projected leaf area in the drought and recovery phases. Vertical and horizontal lines represent 95 % confidence intervals (n = 4–14 for water withholding, n = 4–12 for control).
Fig. 7.
Growth responses of five arabidopsis accessions and the ga5 mutant to water withholding. The traits illustrated are projected leaf area (PLA) (A, C, E, G) and relative growth rate (RGR) (B, D, F, H). RGR was estimated using PLA values. Plants were grown in soil on pots. Grey shading indicates the period of water withholding. Data are mean ± s.e. (n = 4–14 for water withholding, n = 4–12 for control).
Taken together, these data show that this set of ga5 alleles does not yield phenotypes that differ substantially in their response to reduced water availability compared with the corresponding wild-types, and therefore this mutation has no detrimental effect during water limitation. Both semi-dwarf and wild type genotypes maintain growth under reduced water supply, thus pointing to the involvement of additional loci in this response.
DISCUSSION
GA5 is a GA biosynthesis gene with a major effect only on plant height and without other major pleiotropic effects, as observed in mutants in the early steps of the GA pathway (Koornneef and van der Veen, 1980). Among all five AtGA20ox paralogues, GA5 is the only one having a major effect on plant height when mutated, due to its expression pattern in comparison with that of the paralogues (Rieu et al., 2008; Plackett et al., 2012). Because the ga5 mutants show no obvious trade-offs, we can explain why natural variants carrying loss-of-function alleles can be maintained in nature in contrast to mutations in early GA biosynthesis genes such as ga1 (Barboza et al., 2013). Redundancy in the GA20ox genes might maintain GA homeostasis in all organs except the growing inflorescence stem and leaves, thus allowing ga5 mutants to maintain a root system similar to that of the corresponding wild types. Despite this, the ga5 mutant shows a moderate reduction of root length compared with the wild type, an effect observed in previous studies on the Landsberg erecta background (Vartanian et al., 1994), but not in Col (Rieu et al., 2008). This points to the genetic background playing a role, thus suggesting epistasis. A good example has been shown for the SVP gene, for which the same mutation has a different effect on flowering time depending on the genetic background (Méndez-Vigo et al., 2013).
Natural variation in root system architecture has been reported in arabidopsis. Several studies have used QTL analysis to map loci involved in root-related traits (Mouchel et al., 2004; Loudet et al., 2005; Fitz Gerald et al., 2005; Sergeeva et al., 2006). Other studies yielded mapping under different soil mineral concentrations showing the plasticity of the root system and the loci controlling these effects (Prinzenberg et al., 2010; Kellermeier et al., 2013). Genome-wide association studies (GWAS) showed that the genes PHOSPHATE 1 (PHO1) and Root System Architecture 1 (RSA1) were associated with root system allometry (Rosas et al., 2013). However, GWAS for total root length did not result in significant associations, suggesting the involvement of many loci in the control of this trait (Rosas et al., 2013). Another study of natural variation for root length identified loss-of-function alleles in the positive regulator of auxin signalling BRX, which modifies root length under acidic conditions (Gujas et al., 2012). These examples illustrate the role of specific root system architecture traits that may be selected for depending on the environment, as illustrated in a recent review (Lynch, 2013). This plasticity can affect different genes from independent pathways. The possible locus (loci) controlling RSD in the Pak-3 accession has not been mapped and therefore no conclusion can be drawn about whether the variation is due to known pathways or genes. The different root systems found in other Central Asian accessions, such as Kas-2 (relatively short root system) and Neo-3 (relatively large root system width), suggest that the variation present in this region and possible variable selective pressure may allow the evolution of specialized root systems. Our QTL analysis using the Ler × Kas-2 RIL mapping population did not show that ga5 controlled SFW or RSD. However, our QTL profile showed co-localization with QTLs mapped in previous studies using the same population for similar traits (Prinzenberg et al., 2010). The ERECTA locus in chromosome 2 may be a candidate controlling the main QTL for SFW, in agreement with previous studies (Prinzenberg et al., 2010). ERECTA encodes a receptor-like kinase (Torii et al., 1996) and loss-of-function alleles display reduced plant height, a compact inflorescence and rounder and smaller leaves, among other traits (Torii et al., 1996; van Zanten et al., 2009). Regarding the main QTL controlling RSD located on chromosome 4, Prinzenberg et al. (2010) mentioned that a sodium transporter gene located in this region may be implicated, although to our knowledge this has not yet been tested.
The successful use of semi-dwarfs in crops has been a topic for discussion regarding possible trade-offs under drought conditions. The rice variety IR64, carrying mutations in GA20ox2 (Sasaki et al., 2002; Spielmeyer et al., 2002), the functional orthologue of ga5 (GA20ox1), has been reported as drought-sensitive because it was bred for irrigated agricultural environments (Lafitte et al., 2007; Swamy et al., 2013). However, previous studies highlighted the occurrence of rice semi-dwarfs with drought tolerance (Lafitte et al., 2007). The physiological hypothesis behind this might be related to GA–ABA antagonism, whereby semi-dwarfs carrying low GA levels show ABA accumulation, which is beneficial under drought (Lafitte et al., 2007). As found in this study for arabidopsis, using PLA measurements in pot experiments under water-withholding conditions, semi-dwarf accessions may be tolerant (e.g. Pak-3). Even the isogenic comparison between GA20ox mutants, on both the Landsberg erecta and the Col background, show no trade-offs during drought.
Recent studies point to the relevance of RSD in increasing drought avoidance. Natural variants of rice with a deep rooting system have been isolated and the QTL associated with this trait has been identified as DEEPER ROOTING 1 (DRO1) (Uga et al., 2013). Introgression of this gene into cultivated varieties conferred drought resistance. However, it is still disputed whether or not a long root system is directly translated into drought tolerance, as is argued for rice (Lafitte et al., 2007). A deep root system can be useful in soils containing deep water reservoirs. In this study we addressed the possible link between water-limiting conditions and rooting depth, such a link may be found in Pak-3, a semi-dwarf accession with a deep root system that showed high performance under water-limiting conditions. There is still the need to further understand and characterize the possible link between a long root system and its plausible selective advantage in nature and whether it displays possible trade-offs.
In conclusion, semi-dwarfism in arabidopsis does not show pleiotropic effects at the level of the root system that can be attributed to loss-of-function GA5 alleles. We also conclude that there is no apparent positive advantage of semi-dwarfism under water-limiting conditions.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Table S1: growth percentage under sorbitol treatment (relative to control) among arabidopsis GA biosynthesis mutants for different shoot- and root-related traits. Fig. S1: phenotypes of arabidopsis GA biosynthesis mutants. Fig. S2: principal components analysis and correlations for shoot- and root-related traits. Fig. S3: principal components analysis and correlations for shoot- and root-related traits. Fig. S4: frequency distribution for shoot fresh weight and root system depth in the Ler × Kas-2 mapping population grown in vitro. Fig. S5: mean values of shoot fresh weight and root system depth in the Ler × Kas-2 mapping population sorted by the parental alleles using the markers ERECTA, GA5 and SNP304. Fig. S6: QTL maps for shoot fresh weight and root system depth in the Ler × Kas-2 mapping population grown in vitro. Fig. S7: long Pak-3 root system depth occurs independently of the ga5 inactive allele. Fig. S8: osmotic stress response among arabidopsis accessions.
ACKNOWLEDGEMENTS
We thank Tai Ping Sun (Duke University) for providing the ga1-3 6xbxcol, ga3ox1-3 and ga3ox1-3 ga3ox2-1 mutants and Peter Hedden (Rothamsted Research) for the ga20ox1-3 and ga20ox2-1 and ga20ox1 ga20ox2 mutants. L.B. was supported by an International Max Planck Research School PhD Fellowship (IMPRS). The experiments conducted at IBG-2, Forschungszentrum Jülich GmbH were institutionally funded by the Helmholtz Association.
LITERATURE CITED
- Alonso JM, Stepanova AN, Leisse TJ, et al. 2003. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657. [DOI] [PubMed] [Google Scholar]
- Arends D, Prins P, Jansen RC, Broman KW. 2010. R/qtl: high-throughput multiple QTL mapping. Bioinformatics 26: 2990–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barboza L, Effgen S, Alonso-Blanco C, et al. 2013. Arabidopsis semidwarfs evolved from independent mutations in GA20ox1, ortholog to green revolution dwarf alleles in rice and barley. Proceedings of the National Academy of Sciences of the USA 110: 15818–15823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidadi H, Yamaguchi S, Asahina M, Satoh S. 2010. Effects of shoot-applied gibberellin/gibberellin-biosynthesis inhibitors on root growth and expression of gibberellin biosynthesis genes in Arabidopsis thaliana. Plant Root 4: 4–11. [Google Scholar]
- Broman KW, Wu H, Sen S, Churchill GA. 2003. R/qtl: QTL mapping in experimental crosses. Bioinformatics 19: 889–890. [DOI] [PubMed] [Google Scholar]
- Canty A, Ripley B. 2014. boot: Bootstrap R (S-Plus) functions. R package version 1.3-13. [Google Scholar]
- Chloupek O, Forster BP, Thomas WTB. 2006. The effect of semi-dwarf genes on root system size in field-grown barley. Theoretical and Applied Genetics 112: 779–786. [DOI] [PubMed] [Google Scholar]
- Ekstrom C. 2014. MESS: Miscellaneous Esoteric Statistical Scripts. R package version 0.2-1. [Google Scholar]
- Elias AA, Busov VB, Kosola KR, et al. 2012. Green revolution trees: semidwarfism transgenes modify gibberellins, promote root growth, enhance morphological diversity, and reduce competitiveness in hybrid poplar. Plant Physiology 160: 1130–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Lithy ME, Bentsink L, Hanhart CJ, et al. 2005. New Arabidopsis recombinant inbred line populations genotyped using SNPWave and their use for mapping flowering-time quantitative trait loci. Genetics 172: 1867–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz Gerald JN, Lehti-Shiu MD, Ingram PA, Deak KI, Biesiada T, Malamy JE. 2005. Identification of quantitative trait loci that regulate Arabidopsis root system size and plasticity. Genetics 172: 485–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Genuchten MT. 1980. A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Science Society of America Journal 44: 892–898. [Google Scholar]
- Ghanem ME, Hichri I, Smigocki AC, et al. 2011. Root-targeted biotechnology to mediate hormonal signalling and improve crop stress tolerance. Plant Cell Reports 30: 807–823. [DOI] [PubMed] [Google Scholar]
- Gou J, Strauss SH, Tsai CJ, et al. 2010. Gibberellins regulate lateral root formation in Populus through interactions with auxin and other hormones. Plant Cell 22: 623–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujas B, Alonso-Blanco C, Hardtke CS. 2012. Natural Arabidopsis brx loss-of-function alleles confer root adaptation to acidic soil. Current Biology 22: 1962–1968. [DOI] [PubMed] [Google Scholar]
- Hedden P. 2003. The genes of the Green Revolution. Trends in Genetics 19: 5–9. [DOI] [PubMed] [Google Scholar]
- Hedden P, Thomas SG. 2012. Gibberellin biosynthesis and its regulation. Biochemical Journal 444: 11–25. [DOI] [PubMed] [Google Scholar]
- Jansen M, Gilmer F, Biskup B, et al. 2009. Simultaneous phenotyping of leaf growth and chlorophyll fluorescence via GROWSCREEN FLUORO allows detection of stress tolerance in Arabidopsis thaliana and other rosette plants. Functional Plant Biology 36: 902–914. [DOI] [PubMed] [Google Scholar]
- Jia Q, Zhang X-Q, Westcott S, et al. 2011. Expression level of a gibberellin 20-oxidase gene is associated with multiple agronomic and quality traits in barley. Theoretical and Applied Genetics 122: 1451–1460. [DOI] [PubMed] [Google Scholar]
- Karley AJ, Valentine TA, Squire GR. 2011. Dwarf alleles differentially affect barley root traits influencing nitrogen acquisition under low nutrient supply. Journal of Experimental Botany 62: 3917–3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellermeier F, Chardon F, Amtmann A. 2013. Natural variation of Arabidopsis root architecture reveals complementing adaptive strategies to potassium starvation. Plant Physiology 161: 1421–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, van der Veen JH. 1980. Induction and analysis of gibberellin sensitive mutants in Arabidopsis thaliana (L.) Heynh. Theoretical and Applied Genetics 58: 257–263. [DOI] [PubMed] [Google Scholar]
- Koornneef M, Jorna ML, Brinkhorst-van der Swan DLC, Karssen CM. 1982. The isolation of abscisic acid (ABA) deficient mutants by selection of induced revertants in non-germinating gibberellin sensitive lines of Arabidopsis thaliana (L.) Heynh. Theoretical and Applied Genetics 61: 385–393. [DOI] [PubMed] [Google Scholar]
- Koornneef M, Elgersma A, Hanhart CJ, van Loenen-Martinet EP, van Rijn L, Zeevaart JAD. 1985. A gibberellin insensitive mutant of Arabidopsis thaliana. Physiologia Plantarum 65: 33–39. [Google Scholar]
- Lafitte HR, Yongsheng G, Yan S, Li Z-K. 2007. Whole plant responses, key processes, and adaptation to drought stress: the case of rice. Journal of Experimental Botany 58: 169–175. [DOI] [PubMed] [Google Scholar]
- Lê S, Josse J, Husson F. 2008. FactoMineR: An R package for multivariate analysis. Journal of Statistical Software 25: 1–18. [Google Scholar]
- Loudet O, Gaudon V, Trubuil A, Daniel-Vedele F. 2005. Quantitative trait loci controlling root growth and architecture in Arabidopsis thaliana confirmed by heterogeneous inbred family. Theoretical and Applied Genetics 110: 742–753. [DOI] [PubMed] [Google Scholar]
- Luo Y, Dong X, Yu T-Y, et al. 2015. A single nucleotide deletion in GA20ox1 causes alpine dwarfism in Arabidopsis thaliana. Plant Physiology, in press http://dx.doi.org/10.1104/pp.15.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. 2013. Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Annals of Botany 112: 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méndez-Vigo B, Martínez-Zapater JM, Alonso-Blanco C. 2013. The flowering repressor SVP underlies a novel Arabidopsis thaliana QTL interacting with the genetic background. PLoS Genetics 9: e1003289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mendiburu F. 2014. Agricolae: statistical procedures for agricultural research. R package version 1.2-1. [Google Scholar]
- Mitchum MG, Yamaguchi S, Hanada A, et al. 2006. Distinct and overlapping roles of two gibberellin 3-oxidases in Arabidopsis development. Plant Journal 45: 804–818. [DOI] [PubMed] [Google Scholar]
- Mouchel CF, Briggs GC, Hardtke CS. 2004. Natural genetic variation in Arabidopsis identifies BREVIS RADIX, a novel regulator of cell proliferation and elongation in the root. Genes & Development 18: 700–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel KA, Kastenholz B, Jahnke S, et al. 2009. Temperature responses of roots: impact on growth, root system architecture and implications for phenotyping. Functional Plant Biology 36: 947–959. [DOI] [PubMed] [Google Scholar]
- Nagel KA, Putz A, Gilmer F, et al. 2012. GROWSCREEN-Rhizo is a novel phenotyping robot enabling simultaneous measurements of root and shoot growth for plants grown in soil-filled rhizotrons. Functional Plant Biology 39: 891–904. [DOI] [PubMed] [Google Scholar]
- Overvoorde P, Fukaki H, Beeckman T. 2010. Auxin control of root development. Cold Spring Harbor Perspectives in Biology 2: a001537–a001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plackett ARG, Powers SJ, Fernandez-Garcia N, et al. 2012. Analysis of the developmental roles of the Arabidopsis gibberellin 20-oxidases demonstrates that GA20ox1, -2, and -3 are the dominant paralogs. Plant Cell 24: 941–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt A, Horton M, Huang YS, et al. 2010. The scale of population structure in Arabidopsis thaliana. PLoS Genetics 6: e1000843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinzenberg AE, Barbier H, Salt DE, Stich B, Reymond M. 2010. Relationships between growth, growth response to nutrient supply, and ion content using a recombinant inbred line population in Arabidopsis. Plant Physiology 154: 1361–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . 2014. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Rieu I, Ruiz-Rivero O, Fernandez-Garcia N, et al. 2008. The gibberellin biosynthetic genes AtGA20ox1 and AtGA20ox2 act, partially redundantly, to promote growth and development throughout the Arabidopsis life cycle. Plant Journal 53: 488–504. [DOI] [PubMed] [Google Scholar]
- Rosas U, Cibrian-Jaramillo A, Ristova D, et al. 2013. Integration of responses within and across Arabidopsis natural accessions uncovers loci controlling root systems architecture. Proceedings of the National Academy of Sciences of the USA 110: 15133–15138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamini F. 2003. Plant biology. Hormones and the green revolution. Science 302: 71–72. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Ashikari M, Ueguchi-Tanaka M, et al. 2002. Green revolution: a mutant gibberellin-synthesis gene in rice. Nature 416: 701–702. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeeva LI, Keurentjes JJ, Bentsink L, et al. 2006. Vacuolar invertase regulates elongation of Arabidopsis thaliana roots as revealed by QTL and mutant analysis. Proceedings of the National Academy of Sciences of the USA 103: 2994–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielmeyer W, Ellis MH, Chandler PM. 2002. Semidwarf (sd-1), “green revolution” rice, contains a defective gibberellin 20-oxidase gene. Proceedings of the National Academy of Sciences of the USA 99: 9043–9048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T. 2008. Gibberellin metabolism, perception and signaling pathways in Arabidopsis. The Arabidopsis Book 64: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamy BPM, Ahmed HU, Henry A, et al. 2013. Genetic, physiological, and gene expression analyses reveal that multiple QTL enhance yield of rice mega-variety IR64 under drought. PLoS ONE 8: e62795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto E. 2012. Tall or short? Slender or thick? A plant strategy for regulating elongation growth of roots by low concentrations of gibberellin. Annals of Botany 110: 373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii KU, Mitsukawa N, Oosumi T, et al. 1996. The Arabidopsis ERECTA gene encodes a putative receptor protein kinase with extracellular leucine-rich repeats. Plant Cell 8: 735–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uga Y, Sugimoto K, Ogawa S, et al. 2013. Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nature Genetics 45: 1097–1102. [DOI] [PubMed] [Google Scholar]
- Vartanian N, Marcotte L, Giraudat J. 1994. Drought rhizogenesis in Arabidopsis thaliana. Plant Physiology 104: 761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter A, Scharr H, Gilmer F, et al. 2007. Dynamics of seedling growth acclimation towards altered light conditions can be quantified via GROWSCREEN: a setup and procedure designed for rapid optical phenotyping of different plant species. New Phytologist 174: 447–455. [DOI] [PubMed] [Google Scholar]
- Xu Y, Li L, Wu K, Peeters A, Gage D, Zeevaart J. 1995. The GA5 locus of Arabidopsis thaliana encodes a multifunctional gibberellin 20-oxidase: molecular cloning and functional expression. Proceedings of the National Academy of Sciences of the USA 92: 6640–6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S. 2008. Gibberellin metabolism and its regulation. Annual Review of Plant Biology 59: 225–251. [DOI] [PubMed] [Google Scholar]
- Van Zanten M, Snoek LB, Proveniers MCG, Peeters AJM. 2009. The many functions of ERECTA. Trends in Plant Science 14: 214–218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.