Abstract
Variability in placental chorionic surface vessel networks (PCSVNs) may mark developmental and functional changes in fetal health. Here we report a protocol of manually tracing PCSVNs from digital 2D images of post-delivery placentas and its validation by a shape matching method to compare the similarity between paint-injected and unmanipulated (uninjected and deflated vessels) tracings of PCSVNs. We show that tracings of unmanipulated vessels produce networks that are very comparable to the networks obtained by tracing paint-injected PCSVNs. We suggest that manual tracings of unmanipulated PCSVNs can extract features of PCSVN growth and structure that may impact fetal wellbeing.
Keywords: Shape matching, Placental chorionic surface vessel network (PCSVN), shape context, placenta
Introduction
The placental chorionic surface vascular network (PCSVN) develops early in gestation and is critical to fetal wellbeing [1,2]. Current techniques [3-8] of post-delivery 2D and 3D analysis of human placental vasculature are costly, time-consuming, and error-prone.
Paint injection in placental arteries and veins has been used to highlight PCSVNs [9], especially in monochorionic twins to identify anastomoses [10]. We hypothesized that manual tracing of PCSVNs from a high quality, glare-free digital image of the placental chorionic surface reproduces their distinguishing structural elements. We have customized a shape matching algorithm to quantify the similarity between PCSVN tracings before and after paint-injection.
Materials and Methods
Materials
19 singleton placentas delivered at >37 completed weeks were collected. PCSVNs were photographed before (Figure 1a) and after paint injection (Figure 1b). Arterial and venous PCSVNs of each image were traced (Figure 1c) using GNU Image Manipulation Program (GIMP). The manual tracing protocol traces vessel paths using colors to represent specific pixel widths (odd iterations between 3 and 19 pixels), annotating vessel diameters on the 2D image. The 38 tracings were loaded on a custom shape matching Matlab program.
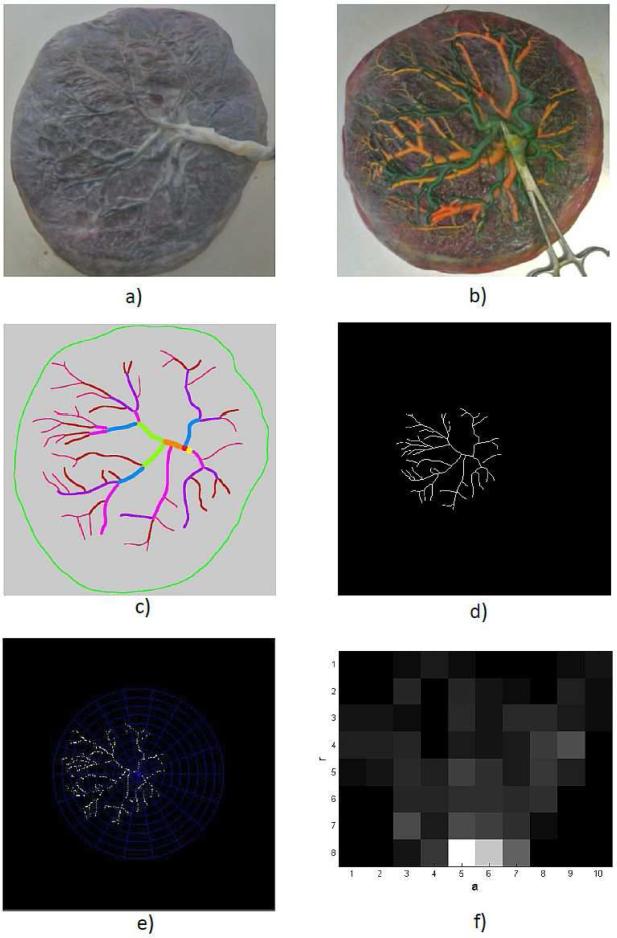
Figure 1. Illustrations of the sequence of events in shape matching algorithm.
a) Glare-free 2D image of a fixed placenta. b) 2D image of placenta with colors injected in arteries and veins. c) Manual tracing of arterial network from uninjected image in a). d) Scaled, translated and skeletonized binary image of manual tracing. e) Visual representation of polar mesh placed on sampled skeletonized image to obtain shapematrix. f) A sample shapematrix with 10 angular bins on horizontal axis and 8 radial bins on vertical axis. Darker bins represent lesser points.
Pre-processing
Tracings are scaled to a 500×500 binary image (1 pixel = 0.1cm), which permits most placentas to fit on the scaled image. Next, each tracing is translated to align its cord insertion point with the center of the binary image (Figure 1d), ensuring that all tracings have a common origin. A single-pixel wide skeleton is extracted that characterizes PCSVN shape and orientation.
Shapematrix computation
Shape matrices were formed on the principle of shape contexts used for shape matching [11] by counting the number of points in various bins of a uniform polar mesh (refer to Supplementary Method for a more detailed explanation). Each skeleton is sampled to retain PCSVN shape and consists of a fixed number of points (n). A polar mesh with ‘a’ angular and ‘r’ radial bins is placed at the center of each image (Figure 1e). We discovered that paint injection distorts PCSVN shape and the placental chorionic surface itself. Uniform radial and angular increments give equal weight to every vascular point irrespective of its distance from the center. The number of points that fall in each bin are counted and an ‘r × a’ shape descriptor for each image is computed (Figure 1f).
Shape matching
We computed the shape matrix of each injected PCSVN and saved them as a training database. The shape matrix of each uninjected PCSVN was computed and then compared against all the images of the training set (See Supplementary Figure 1 to see difference between shapematrices of two different tracings). A shape matching rank was assigned to all training images for each test image, based on the minima of the number of bins in histograms that exceed error threshold ‘e’. Error was computed both intra-pair and inter-pair. For each testing pair of histograms, we shifted the columns of the training histogram ‘a – 1’ times to find a minimum error. This was necessary because the orientations of placental images frequently varied before and after the injection procedure. The minimum intra-pair error was compared to minimum intra-pair errors of other pairs. Finally, the inter-pair error comparison was used to rank the five best matching injected vascular tracings for each uninjected vascular tracing.
Results and Discussion
For each of the 38 uninjected tracings, the algorithm selected the exact injected match with an accuracy of 63% (24 out of 38 tracings matched exactly, see Supplementary Table 1). Injecting paint inside a deflated vessel often modifies its morphology and tortuosity. Additionally, the paint has to be manually milked to advance it into the more distal PCSVN. This random intervention alters the shape of some but not all blood vessels, thereby making a point-to-point comparison difficult for the shape matching algorithm. We then tested the algorithm selections overall, and found that the algorithm assigned the exact match in top 3 ranks with an accuracy of 79%. Figure 2 illustrates examples of the best, average and bad tracing pairs in terms of the algorithm.
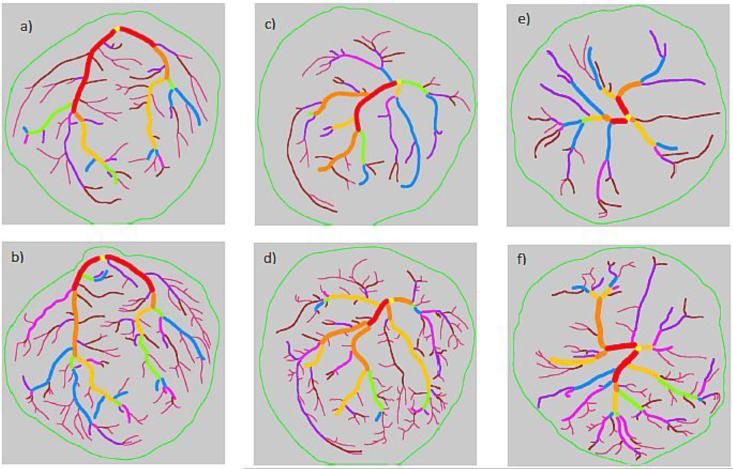
Figure 2. Illustrations of the performance of shape matching algorithm.
The top row (Figures a, c and e) are tracings of uninjected placentas and the bottom row (Figures b, d and e) are their injected counterparts. Figures a) and b) represent an ideal pair that was most frequently matched by the algorithm with minimum error. Figures c) and d) represent an average pair of tracings that was matched by the algorithm with some error. Figures e) and f) represent a pair of tracings that the algorithm found difficult to match.
Most of the injected PCSVN tracings were observed to have more peripheral vessels and vessels with very small diameters. This is obvious; the paint is injected under pressure and will inflates even small ones that are invisible to the naked eye when deflated. To test the effect of tiny vessels on algorithm performance, we eliminated the vessels with the smallest diameter from all injected tracings. With this elimination, the algorithm gave the exact match 71.05% times. Accuracy rose to 84.21% if the correct match was among the top three ranks.
The venous and arterial PCSVNs often travel hand-in-hand to balance the blood flow in and out of a placental lobule. Thus, even minor shape distortion by the manipulation of PCSVN injection may reshape a venous network to resemble the arterial network of the same placenta and vice versa. So we counted how often an injected arterial or venal network of a placenta appeared in the top three matches of a PCSVN of the same placenta. The accuracy was 92.11% when we eliminated the smallest vessels from the injected tracings.
These results support our hypothesis that our manual tracing protocol reliably captures distinct features of the network structure of different PCSVNs. We suggest that manual PCSVN tracings can be used to study relationships between very early placental vascular growth reflected in PCSVNs, and different network structures that might modify placental vascular resistance and/or efficiency, and fetal wellbeing.
Supplementary Material
- Chorionic surface vessel networks were paint injected, photographed and traced
- Pre- and post-injection manual tracings were tested with a shape matching algorithm
- Injected and unmanipulated tracings were frequently matched by the algorithm
- Thinnest vessels on injected tracings were consistently untraceable on uninjected tracings
- Arterial and venous network tracings of same placenta resemble each other the most
Acknowledgement
The project described was supported by The National Children's Study-Placenta Project, LOI 2-BIO-18, Contract Number: HHSN275201100002C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wareing M, Greenwood SL, Fyfe GK, Baker PN. Reactivity of human placental chorionic plate vessels from pregnancies complicated by intrauterine growth restriction (IUGR). Biol Reprod. 2006 Oct;75(4):518–523. doi: 10.1095/biolreprod.106.051607. [DOI] [PubMed] [Google Scholar]
- 2.Wintermark P, Boyd T, Parast MM, Van Marter LJ, War_eld SK, Robertson RL, et al. Fetal placental thrombosis and neonatal implications. Am J Perinatol. 2010 Mar;27(3):251–256. doi: 10.1055/s-0029-1239486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee MM, Yeh MN. Fetal microcirculation of abnormal human placenta. I. Scanning electron microscopy of placental vascular casts from small for gestational age fetus. Am J Obstet Gynecol. 1986 May;154(5):1133–1139. doi: 10.1016/0002-9378(86)90774-x. [DOI] [PubMed] [Google Scholar]
- 4.Lee MM, Yeh MN. Fetal circulation of the placenta: a comparative study of human and baboon placenta by scanning electron microscopy of vascular casts. Placenta. 1983:515–526. 4 Spec No. [PubMed] [Google Scholar]
- 5.Leiser R, Krebs C, Ebert B, Dantzer V. Placental vascular corrosion cast studies: a comparison between ruminants and humans. Microsc Res Tech. 1997;38(1-2):76–87. doi: 10.1002/(SICI)1097-0029(19970701/15)38:1/2<76::AID-JEMT9>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 6.Leiser R, Luckhardt M, Kaufmann P, Winterhager E, Bruns U. The fetal vascularisation of term human placental villi. I. Peripheral stem villi. Anat Embryol (Berl) 1985;173(1):71–80. doi: 10.1007/BF00707305. [DOI] [PubMed] [Google Scholar]
- 7.Pretorius DH, Nelson TR, Baergen RN, Pai E, Cantrell C. Imaging of placental vasculature using three-dimensional ultrasound and color power Doppler: a preliminary study. Ultrasound Obstet Gynecol. 1998 Jul;12(1):45–49. doi: 10.1046/j.1469-0705.1998.12010045.x. [DOI] [PubMed] [Google Scholar]
- 8.Raio L, Ghezzi F, di Naro E, Franchi M, Balestreri D, Dürig P, et al. Inutero characterization of the blood flow in the Hyrtl anastomosis. Placenta. 2001 Jul;22(6):597–601. doi: 10.1053/plac.2001.0685. [DOI] [PubMed] [Google Scholar]
- 9.Gordon Z, Elad D, Almog R, Hazan Y, Jaffa A, Eytan O. Anthropometry of fetal vasculature in the chorionic plate. Journal of anatomy. 2007;211(6):698–706. doi: 10.1111/j.1469-7580.2007.00819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopriore E, Slaghekke F, Middeldorp JM, Klumper FJ, van Lith JM, Walther FJ, et al. Accurate and simple evaluation of vascular anastomoses in monochorionic placenta using colored dye. 2011 doi: 10.3791/3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belongie S, Malik J, Puzicha J. Shape matching and object recognition using shape contexts. Pattern Analysis and Machine Intelligence. IEEE Transactions on. 2002;24(4):509–522. doi: 10.1109/TPAMI.2005.220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.