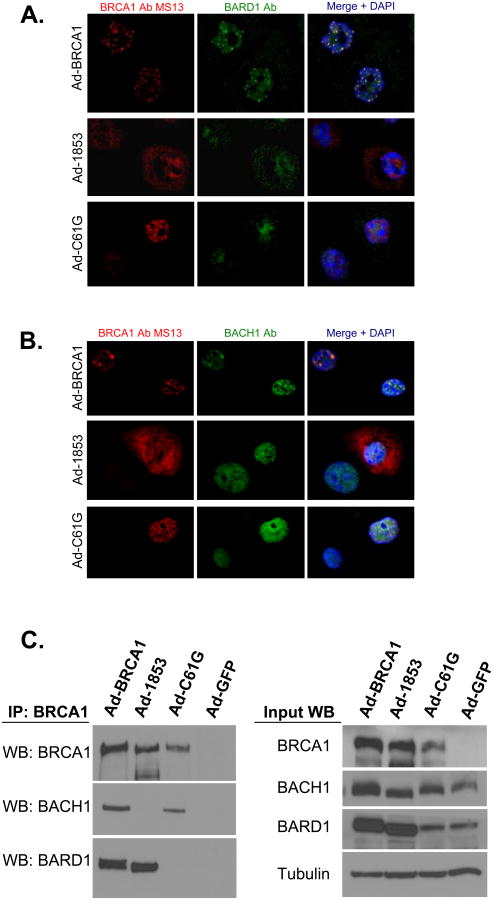
Figure 4. BRCT Domain and RING Domain Mutant BRCA1 Proteins Fail to Co-localize With Both BARD1 and BACH1.
HCC-1937 cells were transduced with adenoviral vectors expressing the specified forms of BRCA1 at MOI=100 and cultured for 48 hours prior to fixation. A) Cells were stained with monoclonal antibody MS13 against BRCA1 (red) and a polyclonal antibody against BARD1 (green). Yellow signal in the merged image indicates co-localization of the two proteins. B) Cells were stained with monoclonal antibody MS13 against BRCA1 (red) and a polyclonal antibody against BACH1 (green). Yellow signal in the merged image indicates co-localization of the two proteins. Nuclei are stained with DAPI in both A and B. Neither C61G (RING mutant) or 1853stop (BRCT mutant) BRCA1 proteins appear to co-localize with either BARD1 or BACH1. Both BARD1 and BACH1 co-localize with wild-type BRCA1. Experiments in A and C were performed three times independently, with similar results. C) Co-immunoprecipitation of BRCA1, BARD1, and BACH1. Lysates were prepared from cells expressing the indicated forms of BRCA1 or control GFP protein and immunoprecipitation of BRCA1 was performed with a combination of monoclonal antibodies (clones MS110 and SD118). Wild-type BRCA1 co-precipitates both BARD1 and BACH1. 1853stop BRCA1 is only capable of interacting with BARD1, while C61G BRCA1 is only capable of interacting with BACH1.