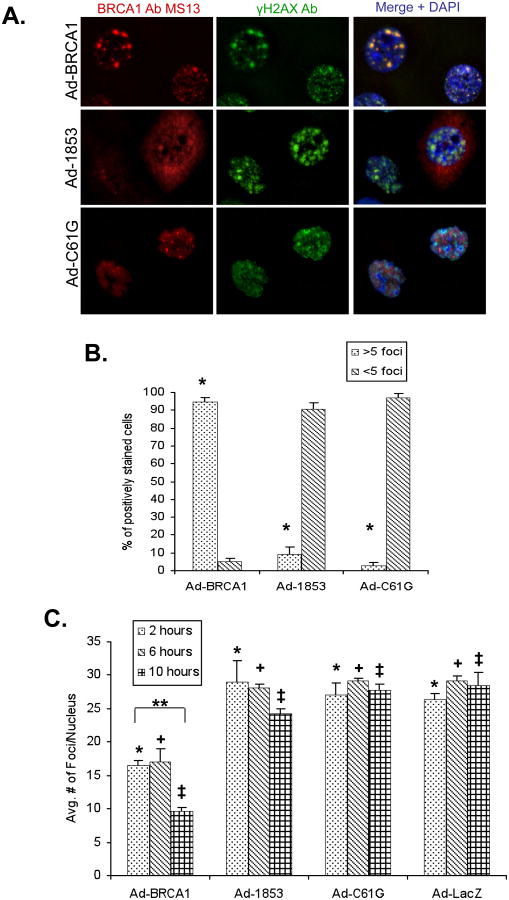
Figure 5. BRCT Domain and RING Domain Mutant BRCA1 Proteins Are Not Effectively Recruited to γH2AX Foci Following Ionizing Radiation.
HCC-1937 cells were transduced at MOI=100 with adenoviral vectors expressing the indicated proteins and cultured for 48 hours prior to treatment. A) Cells expressing the indicated forms of BRCA1 protein were exposed to 2 Gy of ionizing radiation (IR) and cultured for an additional 2 hours at 37°C prior to fixation. Cells were stained with a monoclonal antibody against BRCA1 (red) and a polyclonal antibody against γH2AX (green), which is localized to sites of DNA damage. Wild-type BRCA1 is recruited to γH2AX foci, but both mutant forms of BRCA1 fail to co-localize with γH2AX. The experiment was performed three times. B) Quantification of co-localized BRCA1 and γH2AX foci at 2 hours following 2 Gy IR. 200 cells stained positively for BRCA1 were scored for the number of nuclear foci demonstrating co-localization of BRCA1 and γH2AX proteins. N=3, error bars represent SEM. Wild-type BRCA1 expressing cells consistently showed greater than 5 foci per nucleus, while cells expressing either 1853stop or C61G BRCA1 demonstrated less than 10% of nuclei with greater than 5 foci (*, p<0.001 for both WT vs. 1853stop and WT vs. C61G, student's t-test). C) Quantification of the average number of γH2AX foci per nucleus at 2, 6, and 10 hours post 2 Gy IR. The number of foci per nuclei was counted in 10 representative high powered fields for each group within each experiment (approximately 50 nuclei per group). N=3, error bars represent SEM. The number of foci/nucleus was significantly lower in BRCA1 groups versus 1853stop, C61G, and LacZ at 2 (*), 6 (+), and 10 (‡) hours (p≤0.02 for all comparisons, student's t-test). The decreased number of foci/nucleus in BRCA1 groups between 2 and 10 hours (**) was also significant (p=0.001, student's t-test). The 2 h vs. 10 h comparison in 1853stop groups was not significant.