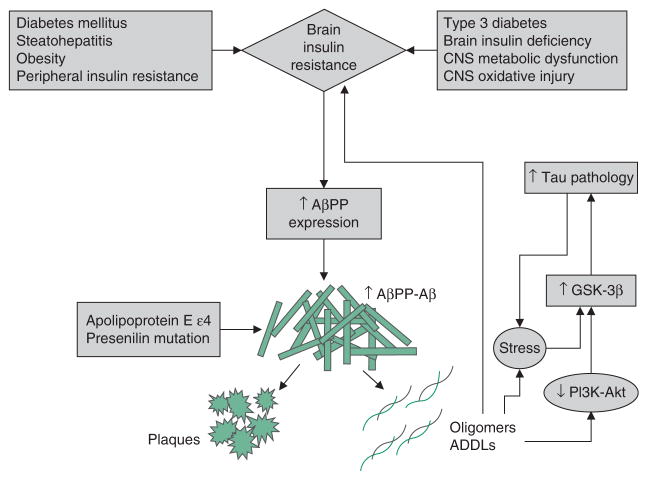
Fig. 1.
Brain insulin resistance and amyloid-β precursor protein (AβPP) Aβ-mediated neurotoxicity. Brain insulin resistance caused by peripheral insulin resistance diseases or intrinsic/genetic processes, toxic exposures or environmental factors contributing to neurodegeneration promote neuroinflammation and increased expression of AβPP. Through the action of β and γ secretases, AβPP is cleaved to generate 40–42 kD AβPP-Aβ peptides that aggregate and form insoluble fibrils and plaques, or oligomers and AβPP-Aβ-derived diffusible ligands (ADDLs), which are neurotoxic. AβPP-Aβ oligomers and ADDLs promote oxidative stress and activate kinases that lead to tau accumulation, hyperphosphorylation and eventual ubiquitination, misfolding and aggregation. AβPP-Aβ oligomers and ADDLs can block insulin-receptor function and contribute to insulin resistance. Carriers of the apoliprotein E ε4 allele or presenilin mutations are predisposed to abnormal AβPP cleavage, and AβPP-Aβ accumulation, aggregation and fibril formation, correlating with increased rates and familial occurrences of Alzheimer’s disease. This scenario depicts a positive feedback or reverberating loop linking AβPP-Aβ ADDL and oligomer accumulation/toxicity with brain insulin resistance, and vice versa. Reproduced from de la Monte,[63] with permission. GSK-3β = glycogen synthase kinase 3β; PI3K= phosphoinositol-3-kinase; ↓ indicates decrease; ↑ indicates increase.