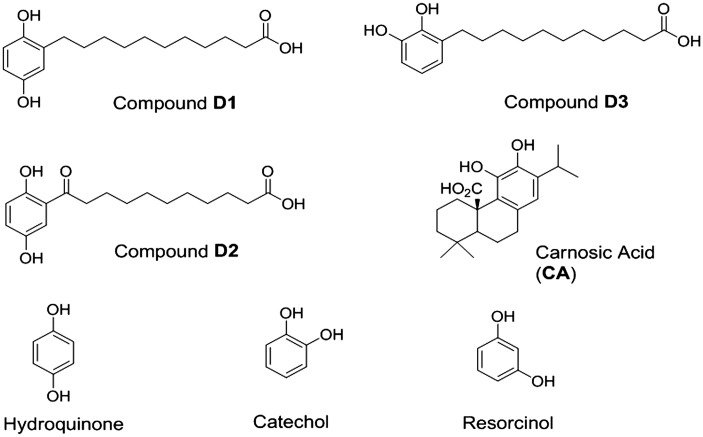
Figure 1.
Chemical structures of the proelectrophiles evaluated in this study. The present study highlights compounds D1 (a) and D3 (b). CA (d) was evaluated as a neuroprotective compound in prior studies and is used here as a reference compound (Satoh et al., 2008a, 2008b). The compound notated as D2 (c) served as an inactive negative control for D1 in previous studies (Satoh et al., 2011). Note that D1 and D2 are para-hydroquinone isomers, while D3 and the natural product CA are ortho-hydroquinone isomers. CA = carnosic acid.