Fig 1. ExoR, ExoS, and ChvI proteins interact to create the RSI Invasion Switch in S. meliloti.
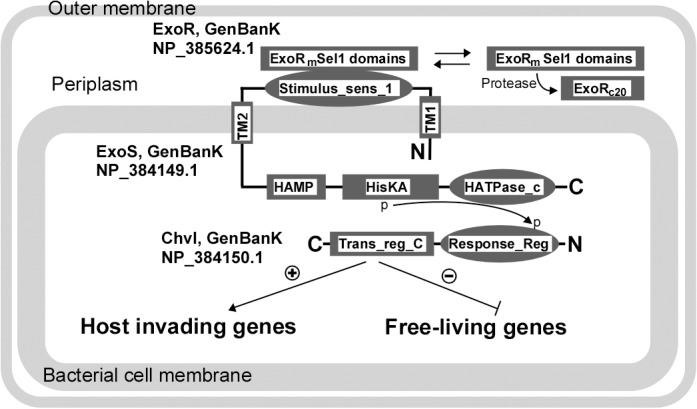
ExoR is composed of Sel1 repeats, is secreted to the periplasm as ExoRm (lacking the signal peptide), and is cleaved to release its suppression of ExoS. The domain architectures of ExoS and ChvI, along with phosphorylation sites, are illustrated here. Signal transmission is achieved via a phosphorylation cascade and ChvI completes the “switch” by binding to DNA to alter gene expression [96]. Our current model suggests that the levels of ExoRm in the periplasm are maintained through the combination of biosynthesis and proteolysis. Proteolysis is possibly sensitive to host signals or to changes in environmental conditions. These mechanisms allow for (1) ExoRm levels to be dramatically reduced in the presence of host signals, (2) the turning “ON” of the RSI invasion switch, (3) the activation of host-invading genes, and (4) the suppression of free-living genes.
