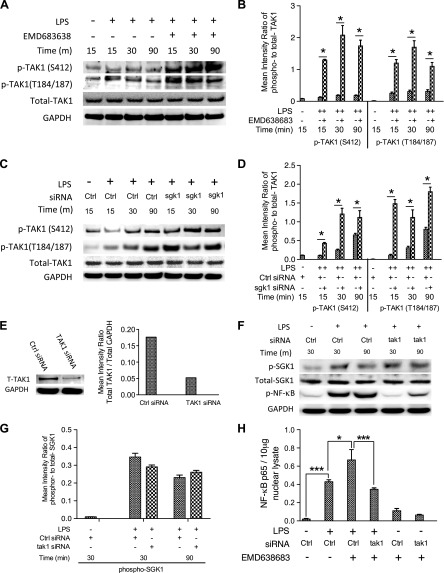
Figure 5.
SGK1 inhibition enhances TLR4-mediated proinflammatory cytokine production dependent on the activity of TAK1. A) Western blot of human monocytes pretreated with EMD638683 for 2 h and then stimulated with LPS. Blots were probed with antibodies to phospho (p)- and total TAK1 and GAPDH as a loading control. B) Densitometric quantification of the mean (sd) ratio of phospho-to-total TAK1 upon LPS stimulation in the presence and absence of EMD638683. C) Western blots of lysates of human monocytes pretreated with nontarget siRNA or specific siRNA targeting SGK1 (as in Fig. 4D), then stimulated with LPS. Blots were probed with antibodies to SGK1, phospho- and total TAK1, and GAPDH as indicated. Data are representative of 3 biologic replicates, and densitometric quantification of the mean (sd) ratio of phospho-to-total TAK1 was performed (D). E) siRNA-mediated knockdown of TAK1 protein and total GAPDH levels were assessed by Western blots. F and G) The effects of siRNA-mediated TAK1 knockdown on the phosphorylation of SGK1 and NF-κB were monitored by Western blots. H) siRNA-mediated TAK1 knockdown and its effects on the DNA binding of NF-κB in nuclear lysates of LPS-stimulated monocytes in the presence and absence of EMD638683. Data represent the arithmetic means ± sd of 3 biologic replicates. *P < 0.05; ***P < 0.001.