Abstract
Context
The extent to which patients take chronic pain medications as prescribed is not well studied, and there are no generally agreed-upon measures. The Quantitative Analgesic Questionnaire (QAQ) is a new instrument designed to comprehensively document patient-reported medication use, generate scores to quantify it (by individual drug, class, and/or overall), and compare it (qualitatively and/or quantitatively) to the regimen as prescribed.
Objectives
The aim of this study was to describe the development and preliminary validation of the QAQ.
Methods
The QAQ was studied in a convenience sample of 149 HIV-infected participants.
Results
We found that the QAQ scores computed for participants’ chronic pain medication regimens were valid based on their correlation with: 1) patient-reported pain intensity (r=0.38, P<0.001); and 2) experienced pain management physicians’ independent quantification of the regimens (r=0.89; P<0.001). The QAQ also demonstrated high inter-rater reliability (r=0.957; P<0.001). Detailed examination of the QAQ data in a subset of 34 participants demonstrated that the QAQ revealed suboptimal adherence in 44% of participants, and contained information that would not have been gleaned from review of the medical record alone in 94%, including use of over-the-counter medications, and quantification of “as needed” dosing. The QAQ also was found to be useful in quantifying change in the medication regimen over time, capturing a change in 50% of the participants from baseline to eight-week follow-up.
Conclusion
The QAQ is a simple tool that can facilitate understanding of patient-reported chronic pain medication regimens, including calculation of percent adherence and generation of quantitative scores suitable for estimating and tracking change in medication use over time.
Keywords: chronic pain, medication adherence, quantitative measures
Introduction
For most chronic illnesses such as diabetes, hypertension or HIV, the concept of medication adherence is fairly straightforward. The goal is that the patient takes 100% of the medication prescribed and anything less is suboptimal. The concept of medication adherence in chronic pain is more complicated because regimens almost always contain elements that are adjusted by the patient according to symptoms, and because of the potential for misuse, particularly with opioids. A broad definition of medication adherence is “the extent to which a patient takes medications as prescribed,”1 but what if variability is part of the prescription? There are currently no generally agreed-upon measures of chronic pain medication adherence. Many studies have used dichotomous outcomes of adherence versus non-adherence based on asking patients whether or not they take their medication exactly as prescribed,2–6 with some studies distinguishing overuse from underuse.7 Others have monitored refills or used electronic pill bottles (medication event monitoring systems, MEMS).8, 9 Each of these approaches has limitations. The dichotomous outcome does not distinguish the patient who misses a single dose from the patient who fails to take the medication altogether, and so has limited clinical significance. Monitoring of medication refills gives little information about how patients actually take their medication. MEMS have the potential to give more detailed information, including percent adherence to individual medications and the regimen overall, but are not practical in most settings.
An alternative conceptual approach to chronic pain medication adherence is to first understand how the patient is actually taking their pain medications, and then compare this “as reported” regimen to the “as prescribed” regimen, accounting for the fact that the “as prescribed” regimen likely contains some medications that are meant to be adjusted by the patient, and others that are not. We sought to design an instrument to operationalize this approach, which would be useful both clinically and in research. To be clinically useful, such an instrument should provide a framework to understand adherence to each prescribed medication, and to discuss and record over-the-counter (OTC) medication use. To be useful in research, such a tool should provide an overall measure of the amount of medication. Herein we describe the development and preliminary validation of such an instrument, the Quantitative Analgesic Questionnaire (QAQ).
Methods
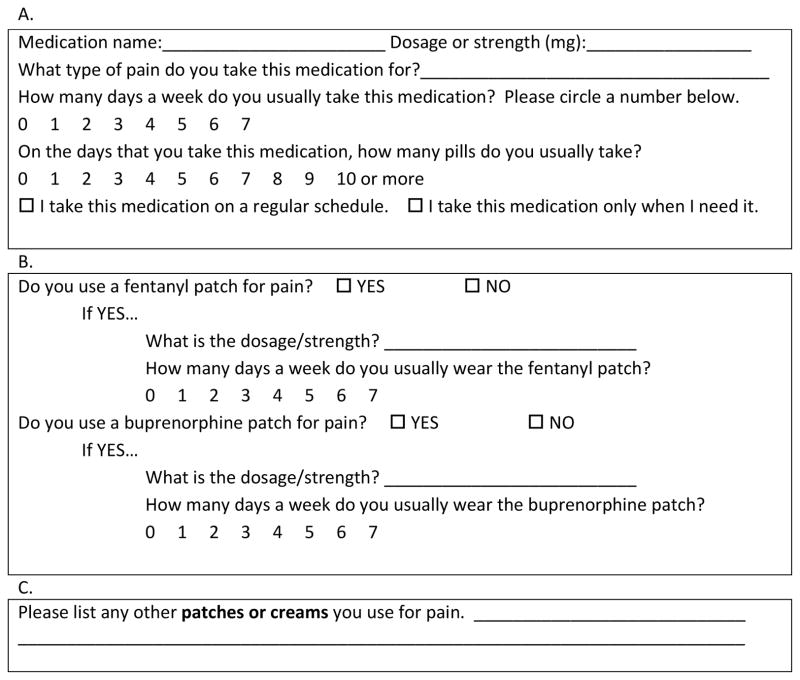
The QAQ comprises a questionnaire and a scoring system. The structure of the questionnaire was adapted from the Brief Medication Questionnaire (BMQ),10 which asks the participant to report the number of days per week a medication was taken and the number of pills per day. The BMQ has been shown to correlate with data obtained from MEMS, 10 and has been used to study adherence in hypertension, HIV and end-stage renal disease.10–13 We modified the BMQ to make it more suitable for use with pain medication regimens, removing a question about “missing pills” because it was not applicable to “as needed” medications, and adding a question to clarify whether each medication was taken on a regular schedule or “as needed.” We also added specific sections for patches and topicals. The resulting QAQ questionnaire has three sections (A, B, and C) as illustrated in Fig. 1. Part A asks the patient to quantify oral medication use in a usual week, part B inquires about use of fentanyl or buprenorphine patches, and part C asks the patient to list any topical agents. In keeping with the BMQ, we queried one week because it is an interval long enough to capture variability, but short enough to facilitate recall. We quantified weekly oral medications by asking the name of the medication; the dosage/strength (medications taken at more than one dose were recorded separately for each dose); how many days a week (0–7) the patient took the medication; and how many pills were usually taken on those days (Fig. 1a). Transdermal opioids (fentanyl and buprenorphine patches) were listed as their own item because of their particular dosing (Fig. 1b). For topicals, we chose just to note their presence because they are difficult to quantify and are a relatively minor part of pain management regimens (Fig. 1c).
Fig. 1.
Sample of sections A, B and C from the Quantitative Analgesic Questionnaire (QAQ).
Similar to the methodology described for the BMQ, the questionnaire was administered by study staff, rather than given to participants to complete on their own. Prior to administration, the patient was informed that the purpose of the questionnaire was to record all medications taken for pain including medications prescribed by a doctor and those available OTC, and that we wished to record how they actually use the medication, even if this differed from the doctor’s instructions or product labeling.
The QAQ was completed in three steps. In the first step, the participant was asked to spontaneously recall pain medications. In the second step, the participant was specifically queried about any additional OTC medications. In the third step, their medication list (derived from the electronic medical record) was reviewed, and the participant was asked specifically about each medication in the list with a potential indication for pain.
In order to use the QAQ to quantify the amount of patient-reported pain medication use, we wanted to develop a scoring system that was conceptually simple, was easily automated using commonly available tools (e.g., a spreadsheet), and could readily accept the addition of new medications. QAQ scores are calculated from the questionnaire as follows:
The total amount of each individual drug taken per week is calculated (dosage x days per week x pills per day).
The values calculated in step 1 for opioids are converted into equivalent mg of oral morphine and summed. The values calculated in step 1 for all other oral medications are converted into percentage of maximum recommended dose according to the product labeling (percentages may be greater or less than 100%).
The values calculated in step 2 are converted into points. One point is assigned for taking any opioids at all, and an additional point is assigned for every equivalent of 100 mg of oral morphine used per week (e.g., 1–99 mg = 1 point; 100–199 = 2 points, etc.). For all other oral medications, one point is assigned for taking the medication at all, and an additional point is assigned for each 25% of the maximum dose taken (e.g., 1–24% = 1 point; 25–49% = 2 points, etc.). For topicals, one point is assigned for each topical the patient reports using (e.g., lidocaine patch) regardless of dose or frequency.
A total score is then generated by summing all the points from step 3. Subscores can be generated for particular classes of medications (e.g., opioids, non-opioids, adjuvants) if desired.
We chose 100 mg of oral morphine per week and 25% of the maximum recommended dosage as our units because they are convenient numbers that reflect meaningful changes. For example, using this system, a one-point change would represent a change of about 15 mg of oral morphine daily or about 10 mg of oxycodone daily. A sample calculation is shown in Fig. 2.
Fig. 2.
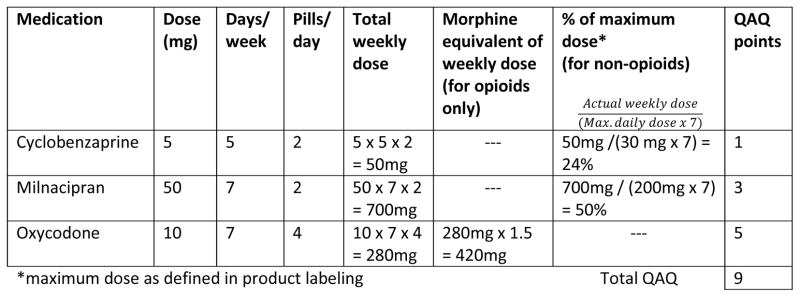
Sample Quantitative Analgesic Questionnaire (QAQ) calculation.
Data for the analyses were obtained from a convenience sample consisting of two groups of participants, referred to as the “retrospective” and “prospective” groups. The retrospective group (n=115) was enrolled in a study of HIV-associated neuropathy as previously described,14, 15 and the prospective group was enrolled in a study of Mindfulness-Based Stress Reduction (MBSR) for the treatment of chronic neuropathic and musculoskeletal pain in HIV (n=34), the results of which have not yet been published. All procedures were performed in accordance with a protocol approved by the institutional review board of the Icahn School of Medicine at Mount Sinai, all participants gave written informed consent.
Statistical Analyses
We assessed the validity of the QAQ score in two ways, both of which used data pooled from the prospective and retrospective samples (n=149). The relevant data included chronic pain medication regimens and patient-reported pain intensities (on a 0–10 numeric rating scale). First, we sought to determine if the QAQ scores calculated from the chronic pain medication regimens were correlated with the clinical impressions of these regimens as reported by physicians with expertise in pain management. In order to do this, we extracted all the pain management regimens from participants in both studies, excluding regimens that were extremely similar. This yielded 56 distinct regimens. Seven clinicians, blinded to the method by which the QAQ score was calculated, were asked to rate each regimen on a scale of 1–5 where one was a small amount of medication and five was a large amount of medication. Clinicians were from a variety of disciplines (anesthesia, neurology, palliative care, rehabilitation medicine and rheumatology) and were all attending physicians who actively treat chronic pain. Our second validation method was to determine whether the QAQ score was correlated with patient-reported pain intensity on a 0–10 numeric pain rating scale. Inter-rater reliability of the QAQ was assessed in the prospective patient group only. Two different members of the study team administered the QAQ to participants on the same day, without knowledge of prior results, and inter-rater reliability was judged based on the strength of the correlation between the two ratings. All correlations were calculated using Spearman’s rank correlation. All statistical tests were two-tailed and conducted at the α = 0.05 level using IBM SPSS Statistics for Windows, v. 20 (IBM Corp., Armonk, NY).
Results
Participants
The demographic characteristics of the retrospective and prospective patient samples were similar. In both samples, the mean age was in the early 50s, the majority of participants were African American or Hispanic (<10% non-Hispanic White in both), and there was a nearly equal distribution of men and women. The sources of pain were varied and included: fibromyalgia, chronic low back pain, osteoarthritis, myofascial pain, osteonecrosis, HIV-associated distal symmetric polyneuropathy, HIV-associated vacuolar myelopathy, and post-herpetic neuralgia. All major classes of pain medications were included among the chronic pain medication regimens (opioids, acetaminophen, non-steroidal anti-inflammatory drugs, tricyclic antidepressants, antiepileptics, and serotonin-norepinephrine reuptake inhibitors.)
Validity and Reliability
The QAQ scores calculated for 56 chronic pain medication regimens were closely aligned with scores assigned by the seven pain management physicians based on clinical impression. This correlation held for each physician individually (r=0.75–0.83; P<0.001 for all correlations), and was even stronger when the physicians’ responses were averaged (r=0.89; P<0.001). A “ceiling effect” was observed in the physician responses, presumably because of the limited (1–5) scoring options, in which the relationship between their scores and the QAQ was relatively flat above a QAQ score of 20. In order to account for this, we also log transformed the QAQ and repeated the correlation, which yielded essentially the same results (r=0.89; P<0.001). The QAQ score also was correlated with the participants’ self-reported pain intensity (r=0.38, P<0.001). The QAQ had high inter-rater reliability, as demonstrated by a strong correlation between the QAQ scores obtained by two separate members of the study team, who administered the tool to the same participants on the same day, each without knowledge of the other’s results (r=0.957; P<0.001).
Descriptives
In 32 of the 34 participants (94%) to whom the QAQ was administered prospectively, the QAQ contained information that would not have been gleaned from review of the medical record alone. The information captured included: OTC medications (22 patients), quantification of the amount of “as needed” dosing (16 patients), discovery of medications prescribed that patients were not taking at all (10 patients), prescribed medications that were taken differently than directed (three patients), and prescription medications that the patient was taking but that did not appear in the medical record (one patient).
The ability of the QAQ to quantify adherence to individual medications was assessed by calculating total weekly doses as prescribed and comparing this value to the total weekly dose captured by the QAQ questionnaire. In the case of “as needed” medications, the total weekly prescribed dose was calculated as a range, and any amount within this range was considered 100% adherence. This procedure revealed that 41% of patients were underusing one or more prescribed medications and 3% were overusing a prescribed medication, with adherence estimates ranging from 0–133%.
The ability of the QAQ to detect change over time was assessed in the prospective participants by administering the QAQ at two time points eight weeks apart. The QAQ detected a change in 50% of the participants, ranging in magnitude from −4 to 10 points.
Discussion
Medication adherence, the extent to which patients take medications as prescribed, is not well studied in chronic pain, and there are no generally agreed upon methods for doing so.7 A systematic review of the topic in 2009 identified 14 studies, with a wide variety of outcome measures.16 Many of these studies, and others published more recently, used a dichotomous outcome (adherent versus non-adherent), with non-adherence defined in various ways including: self-report of any missing doses;3 at least one positive response on the Morisky Medication Adherence Scale (a four- item questionnaire);2 inability to state the medication regimen exactly;4 self-report of not taking the medication exactly as prescribed;5, 7 and in the case of opioids, unexpected urine toxicology results.6 Other studies have used continuous outcomes including: a brief series of questions querying medication overuse or underuse generally (resulting in a continuous measure),17 and percentage adherence based on pill counting,18 tracking medication refills,9 or MEMS.8 These existing methodologies all have limitations. Dichotomous outcomes do not capture any detailed information about how medications are being used and so are of limited clinical relevance, and technology-intensive approaches are not practical in many settings.
In this study we took a conceptually different approach to chronic pain medication adherence. Rather than asking patients whether or not they take their medications exactly as prescribed, we sought to capture and quantify the pain medication regimen “as reported,” and then compare it to the regimen “as prescribed.” This approach has several potential benefits. Asking the patient how they take their medication is more neutral than asking whether they follow their doctor’s instructions, which may increase the likelihood of honest reporting. Detailed qualitative understanding of the “as reported” regimen is valuable clinical information. Quantification of a pain medicine regimen is useful for monitoring change over time. Finally, a quantitative continuous adherence outcome based on patient report is more informative than a dichotomous one, and more practical than a technology-dependent outcome.
The instrument we developed, the QAQ, has two parts: a questionnaire and a scoring system. We demonstrated that the QAQ score has clinical meaning. The QAQ scores for 56 chronic pain management medication regimens were well correlated with how physicians experienced in chronic pain management judged these regimens. The QAQ score also was correlated with patients’ self-reported pain intensity. The QAQ had excellent inter-rater reliability, was able to detect differences between the pain medication regimen “as reported” and “as prescribed,” to quantify pain medication adherence, and to detect change over time.
This study has limitations. Much of the data were retrospective and none of it was acquired with the primary purpose of developing and/or validating the QAQ. Thus we do not have data demonstrating the accuracy of the self-report data as captured by the QAQ questionnaire, such as correlation with MEMS, although the questionnaire from which the QAQ was derived, the BMQ, has been shown to correlate with MEMS.10 All of the patients in this study were HIV-positive and were recruited from a single academic center, thus the prescribing patterns may reflect peculiarities of this particular population and may not have covered the full breadth of regimens calculable using the QAQ. Our patient population is a predominantly minority, inner city population with high rates of medical comorbidity, and low health literacy. Thus the QAQ was administered to them by study staff. The QAQ would likely not perform well as a purely self-report tool in this population, but future studies could explore this issue in other populations, for example by comparing the results of the QAQ filled out by patients alone to the QAQ filled out by patients and staff together. Medication adherence is a subject of intense focus in the treatment of HIV, which may permeate other aspects of the patients’ care including pain management. Also, there are high rates of substance abuse and psychiatric comorbidity in this population; thus patients may be more accustomed to having their therapies closely monitored and, therefore, less likely to deviate from provider instructions, or if they do, to report such deviation. Finally, there are other instruments available to quantify analgesic use across multiple classes of medications, most notably the Medication Quantification Scale (MQS), but such scales are more suitable for use with pharmacy records and do not provide a method for accounting for how patients report actually taking their medication.19–21 In addition, the MQS relies upon a complicated system of detriment weighting that requires periodic survey of large numbers of pain management experts to update, and suggests that conceptually the MQS is a measure of the ability of pain medications to harm rather than treat patients with chronic pain.
In summary the QAQ is a conceptually simple tool that can be used to facilitate understanding of a chronic pain medication regimen as the patient reports taking it, and subsequently can be used to quantify the regimen, estimate percentage adherence and track change over time. Further investigation in other patient populations, and in large clinical trials is needed to demonstrate its usefulness as an outcome measure.
Acknowledgments
This work was supported by a grant (K23 NS066789) from the National Institute of Neurological Disorders and Stroke to Dr. Robinson-Papp, and the CTSA grant (UL1 TR000067) awarded to the Mount Sinai School of Medicine.
The authors thank Dr. James Godbold for statistical consultation.
Footnotes
Disclosures
The authors report no conflicts of interest relevant to the content of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73:691–705. doi: 10.1111/j.1365-2125.2012.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sewitch MJ, Dobkin PL, Bernatsky S, et al. Medication non-adherence in women with fibromyalgia. Rheumatology. 2004;43:648–654. doi: 10.1093/rheumatology/keh141. [DOI] [PubMed] [Google Scholar]
- 3.Weinberger M, Tierney WM, Booher P, Katz BP. The impact of increased contact on psychosocial outcomes in patients with osteoarthritis: a randomized, controlled trial. J Rheumatol. 1991;18:849–854. [PubMed] [Google Scholar]
- 4.Berndt S, Maier C, Schutz HW. Polymedication and medication compliance in patients with chronic non-malignant pain. Pain. 1993;52:331–339. doi: 10.1016/0304-3959(93)90167-N. [DOI] [PubMed] [Google Scholar]
- 5.Timmerman L, Stellema R, Stronks DL, Groeneweg G, Huygen FJ. Adherence to pharmacological pain therapy in patients with non-malignant pain: the role of patients’ knowledge of pain medication. Pain Pract. 2014;14:701–708. doi: 10.1111/papr.12139. [DOI] [PubMed] [Google Scholar]
- 6.Ives TJ, Chelminski PR, Hammett-Stabler CA, et al. Predictors of opioid misuse in patients with chronic pain: a prospective cohort study. BMC Health Serv Res. 2006;6:46. doi: 10.1186/1472-6963-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broekmans S, Dobbels F, Milisen K, Morlion B, Vanderschueren S. Pharmacologic pain treatment in a multidisciplinary pain center: do patients adhere to the prescription of the physician? Clin J Pain. 2010;26:81–86. doi: 10.1097/AJP.0b013e3181b91b22. [DOI] [PubMed] [Google Scholar]
- 8.de Klerk E, van der Linden SJ. Compliance monitoring of NSAID drug therapy in ankylosing spondylitis, experiences with an electronic monitoring device. Br J Rheumatol. 1996;35:60–65. doi: 10.1093/rheumatology/35.1.60. [DOI] [PubMed] [Google Scholar]
- 9.Deyo RA, Inui TS, Sullivan B. Noncompliance with arthritis drugs: magnitude, correlates, and clinical implications. J Rheumatol. 1981;8:931–936. [PubMed] [Google Scholar]
- 10.Svarstad BL, Chewning BA, Sleath BL, Claesson C. The brief medication questionnaire: a tool for screening patient adherence and barriers to adherence. Patient Educ Couns. 1999;37:113–124. doi: 10.1016/s0738-3991(98)00107-4. [DOI] [PubMed] [Google Scholar]
- 11.Choo PW, Rand CS, Inui TS, et al. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Med Care. 1999;37:846–857. doi: 10.1097/00005650-199909000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Curtin RB, Svarstad BL, Keller TH. Hemodialysis patients’ noncompliance with oral medications. ANNA J. 1999;26:307–316. [PubMed] [Google Scholar]
- 13.Rathbun RC, Farmer KC, Stephens JR, Lockhart SM. Impact of an adherence clinic on behavioral outcomes and virologic response in treatment of HIV infection: a prospective, randomized, controlled pilot study. Clin Ther. 2005;27:199–209. doi: 10.1016/j.clinthera.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Robinson-Papp J, Sharma S, Simpson DM, Morgello S. Autonomic dysfunction is common in HIV and associated with distal symmetric polyneuropathy. J Neurovirol. 2013;19:172–180. doi: 10.1007/s13365-013-0160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson-Papp J, Sharma SK. Autonomic neuropathy in HIV is unrecognized and associated with medical morbidity. AIDS Patient Care STDS. 2013;27:539–543. doi: 10.1089/apc.2013.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broekmans S, Dobbels F, Milisen K, Morlion B, Vanderschueren S. Medication adherence in patients with chronic non-malignant pain: Is there a problem? Eur J Pain. 2009;13:115–123. doi: 10.1016/j.ejpain.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Rosser BA, McCracken LM, Velleman SC, Boichat C, Eccleston C. Concerns about medication and medication adherence in patients with chronic pain recruited from general practice. Pain. 2011;152:1201–1205. doi: 10.1016/j.pain.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 18.Mulleners WM, Whitmarsh TE, Steiner TJ. Noncompliance may render migraine prophylaxis useless, but once-daily regimens are better. Cephalalgia. 1998;18:52–56. doi: 10.1046/j.1468-2982.1998.1801052.x. [DOI] [PubMed] [Google Scholar]
- 19.Masters Steedman S, Middaugh SJ, Kee WG, et al. Chronic-pain medications: equivalence levels and method of quantifying usage. Clin J Pain. 1992;8:204–214. [PubMed] [Google Scholar]
- 20.Harden RN, Weinland SR, Remble TA, et al. Medication quantification scale version III: update in medication classes and revised detriment weights by survey of American Pain Society physicians. J Pain. 2005;6:364–371. doi: 10.1016/j.jpain.2005.01.350. [DOI] [PubMed] [Google Scholar]
- 21.Gallizzi M, Gagnon C, Harden RN, Stanos S, Khan A. Medication Quantification Scale version III: internal validation of detriment weights using a chronic pain population. Pain Pract. 2008;8:1–4. doi: 10.1111/j.1533-2500.2007.00163.x. [DOI] [PubMed] [Google Scholar]