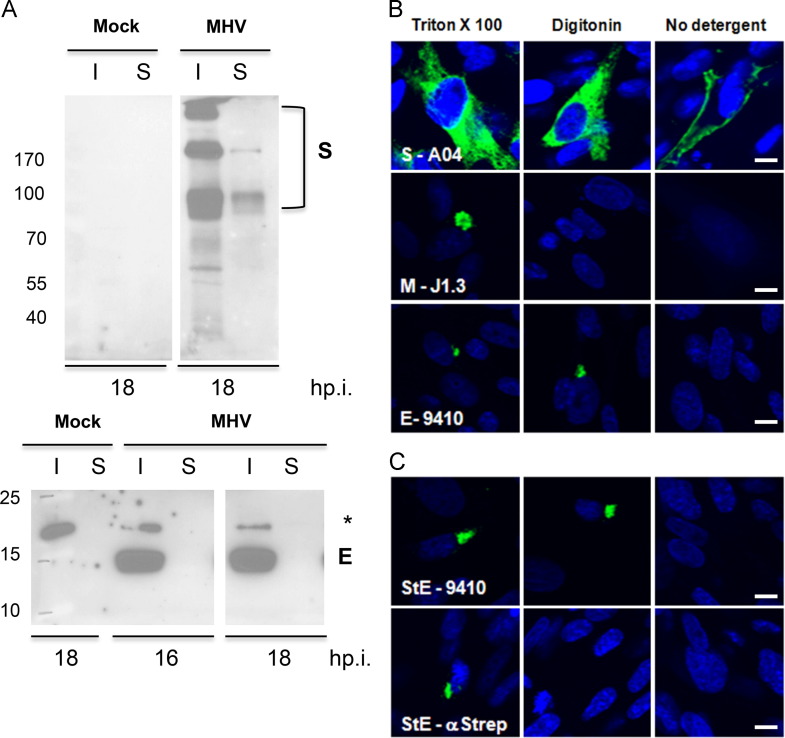
Fig. 3.
MHV E does not traffic to the cell surface. (A) 17Cl1 cells were infected with MHV at an MOI of 0.1. At 16 & 18 h p.i., surface proteins were biotinylated. Surface (S) and intracellular (I) proteins were analyzed for spike (S) and envelope (E) proteins by Western blotting. For the analysis 10% and 20% of the intracellular and surface fractions, respectively were loaded. The Western blot for the E protein was exposed 15× longer than that for the S protein. (B) 17Cl1 mouse cells were infected with MHV at an MOI of 0.1 (top three panels) and probed with antibodies specific for S, M and E after cells were permeabilized with digitonin or TX-100 to reveal the cytoplasmic or both luminal and cytoplasmic epitope of the protein. (C) BHK-21 cells were transfected with pCAGGS vector expressing MHV E with a Strep- tag at the amino end. The amino and carboxy ends of E were detected with an anti-Strep monoclonal, StrepMAB-Classic and rabbit anti-E 9410 antibodies, respectively. Scale bar, 5 μm.