Table 3.
Dehydrogenative cross-coupling of aldehydes and amines.[a]
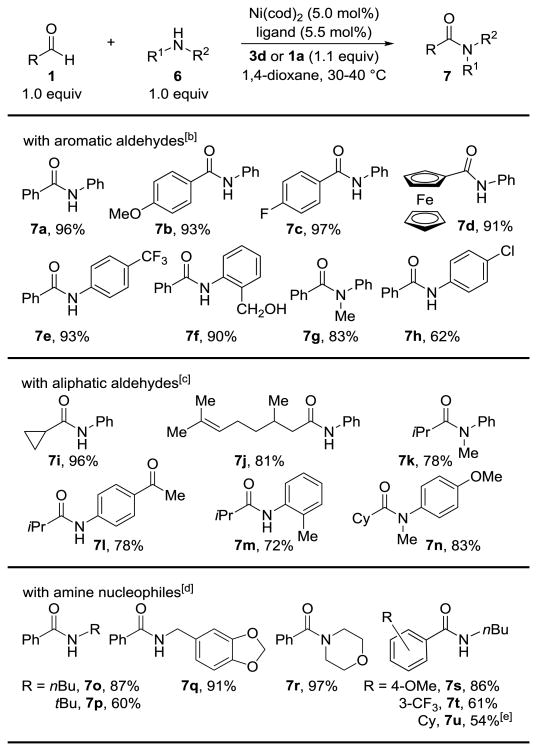
|
Conditions: 0.5 mmol 1, 0.5 mmol 6, 0.2 M, 8 h. Isolated yields are shown.
The reactions were performed at 40 °C using IPr as the ligand and 3d as the hydrogen acceptor.
The reactions were performed at 30 °C using IPr as a ligand and 3d as the hydrogen acceptor.
The reactions were performed at 40 °C using ItBu as the ligand and an extra equivalent of the aldehyde as the hydride acceptor.
Using 1a rather than cyclohexanecarboxaldehyde as the hydrogen acceptor.
