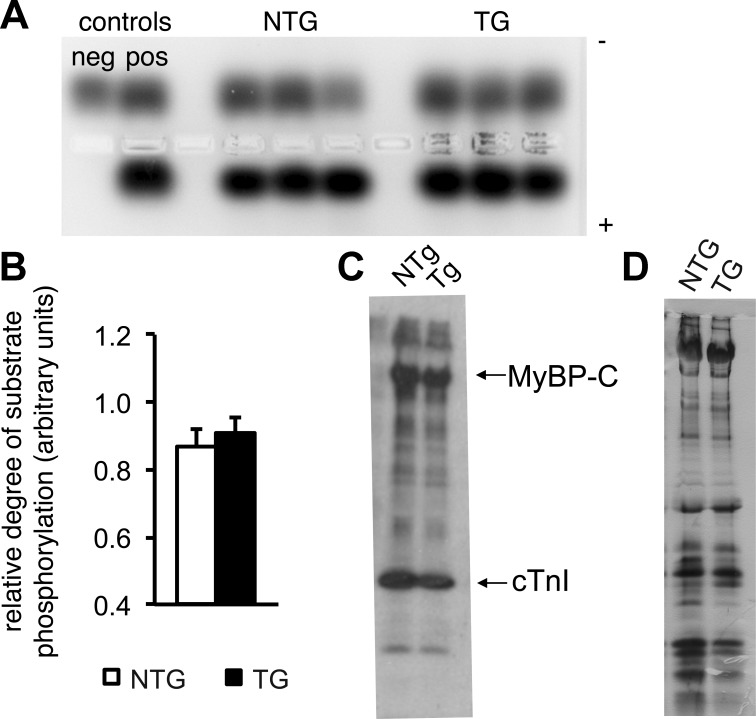
Fig. 5.
PKA activity is normal in Q510E-SHP2-expressing cardiomyocytes. A: ventricular tissue lysates were incubated with a PKA-specific peptide substrate and, subsequently, the degree of substrate phosphorylation was assessed by gel electrophoresis. The phosphorylated species migrated toward the positive electrode, whereas the nonphosphorylated substrate migrated toward the negative electrode. B: quantification of the relative degree of PKA substrate phosphorylation. n = 11 per group. C and D: representative autoradiograms (C) and silver-stained protein gel (D) after PKA backphosphorylation. After normalization for total protein load, the degree of radiolabel incorporation in the various sarcomeric proteins did not differ between cardiomyocytes from NTG versus TG hearts. MyBP-C, myosin-binding protein-C; cTnI, cardiac troponin I. n = 3 per group.