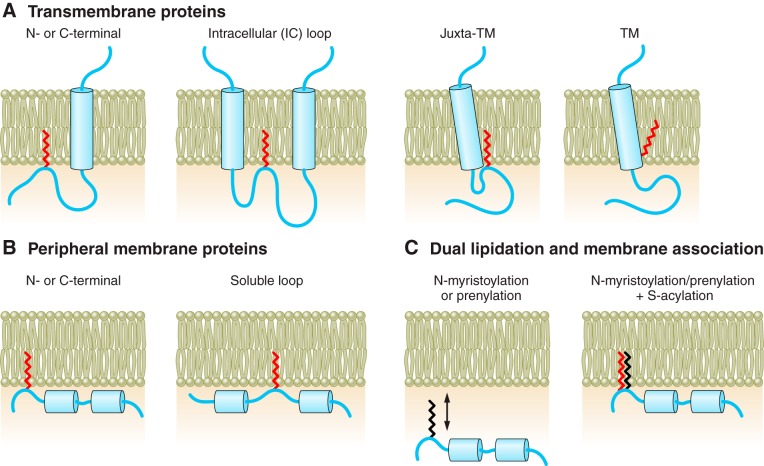
Figure 3.
Location of sites of S-acylation in transmembrane and peripheral-membrane proteins. Schematic illustrating different locations of cysteine S-acylation in transmembrane and peripheral membrane proteins. A: for transmembrane proteins, S-acylation may allow a cytosolic NH2 or COOH terminus or intracellular (IC) loop of a protein to associate with the membrane interface. S-acylation can also confer structural constraints in particular when located close to, or within, transmembrane (TM) domains. In several cases, S-acylation near a TM domain has been proposed to control transmembrane orientation that may be important for controlling hydrophobic mismatch in different subcellular membrane compartments. B: for peripheral membrane proteins, S-acylation of NH2 or COOH terminus or soluble loops may control membrane association of the protein. In many cases, membrane interaction is promoted through other membrane affinity domains (such as hydrophopic or polybasic domains) of the protein. C: alternatively, dual lipidation is required for membrane association including prenylation or myristoylation at sites close to the S-acylated cysteine residue.