Abstract
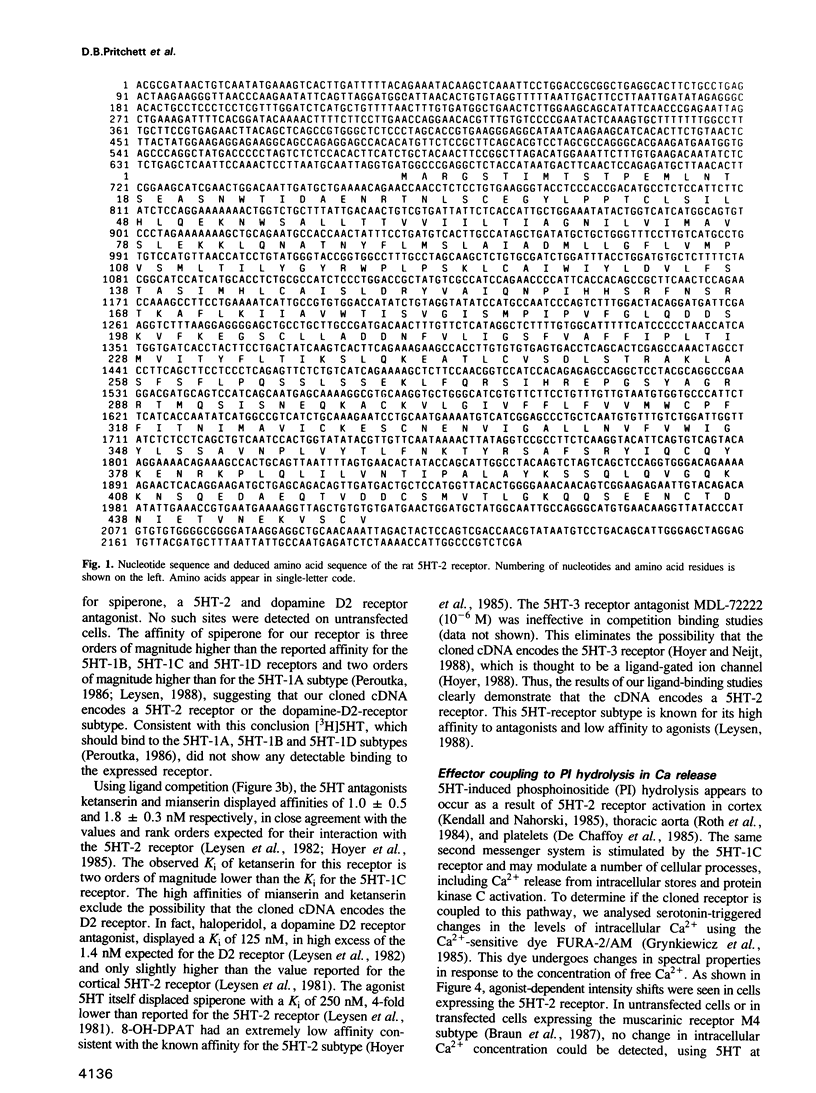
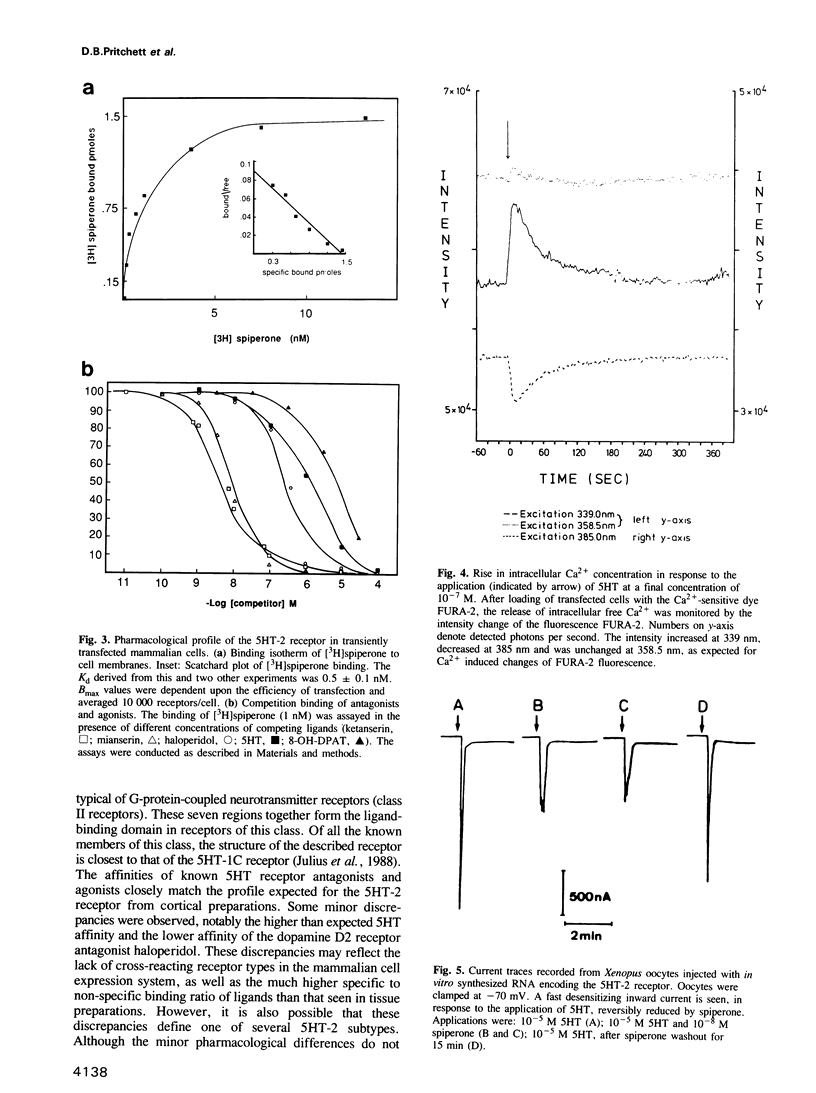
A complementary DNA (cDNA) encoding a serotonin receptor with 51% sequence identity to the 5HT-1C subtype was isolated from a rat brain cDNA library by homology screening. Transient expression of the cloned cDNA in mammalian cells was used to establish the pharmacological profile of the encoded receptor polypeptide. Membranes from transfected cells showed high-affinity binding of the serotonin antagonists spiperone, ketanserin and mianserin, low affinity for haloperidol (a dopamine D2 receptor antagonist), 8-OH-DPAT as well as MDL-72222 and no detectable binding of [3H]serotonin. This profile is consonant with the 5HT-2 subtype of serotonin receptors. In agreement with this assignment, serotonin increased the intracellular Ca2+ concentration and activated phosphoinositide hydrolysis in transfected mammalian cells. The agonist also elicited a current flow, blocked by spiperone, in Xenopus oocytes injected with in vitro synthesized RNA containing the cloned nucleotide sequences.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner T. I., Buckley N. J., Young A. C., Brann M. R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987 Jul 31;237(4814):527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Young A. C., Brann M. R., Buckley N. J. Cloning and expression of the human and rat m5 muscarinic acetylcholine receptor genes. Neuron. 1988 Jul;1(5):403–410. doi: 10.1016/0896-6273(88)90190-0. [DOI] [PubMed] [Google Scholar]
- Bradley P. B., Engel G., Feniuk W., Fozard J. R., Humphrey P. P., Middlemiss D. N., Mylecharane E. J., Richardson B. P., Saxena P. R. Proposals for the classification and nomenclature of functional receptors for 5-hydroxytryptamine. Neuropharmacology. 1986 Jun;25(6):563–576. doi: 10.1016/0028-3908(86)90207-8. [DOI] [PubMed] [Google Scholar]
- Braun T., Schofield P. R., Shivers B. D., Pritchett D. B., Seeburg P. H. A novel subtype of muscarinic receptor identified by homology screening. Biochem Biophys Res Commun. 1987 Nov 30;149(1):125–132. doi: 10.1016/0006-291x(87)91613-5. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N., Landau E. M., Lass Y. Xenopus oocyte resting potential, muscarinic responses and the role of calcium and guanosine 3',5'-cyclic monophosphate. J Physiol. 1984 Jul;352:551–574. doi: 10.1113/jphysiol.1984.sp015310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Kobilka B. K., Strader D. J., Benovic J. L., Dohlman H. G., Frielle T., Bolanowski M. A., Bennett C. D., Rands E., Diehl R. E. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. 1986 May 1;321(6065):75–79. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- Fargin A., Raymond J. R., Lohse M. J., Kobilka B. K., Caron M. G., Lefkowitz R. J. The genomic clone G-21 which resembles a beta-adrenergic receptor sequence encodes the 5-HT1A receptor. Nature. 1988 Sep 22;335(6188):358–360. doi: 10.1038/335358a0. [DOI] [PubMed] [Google Scholar]
- Glennon R. A., Titeler M., McKenney J. D. Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci. 1984 Dec 17;35(25):2505–2511. doi: 10.1016/0024-3205(84)90436-3. [DOI] [PubMed] [Google Scholar]
- Gorman C., Padmanabhan R., Howard B. H. High efficiency DNA-mediated transformation of primate cells. Science. 1983 Aug 5;221(4610):551–553. doi: 10.1126/science.6306768. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hoyer D., Engel G., Kalkman H. O. Molecular pharmacology of 5-HT1 and 5-HT2 recognition sites in rat and pig brain membranes: radioligand binding studies with [3H]5-HT, [3H]8-OH-DPAT, (-)[125I]iodocyanopindolol, [3H]mesulergine and [3H]ketanserin. Eur J Pharmacol. 1985 Nov 26;118(1-2):13–23. doi: 10.1016/0014-2999(85)90658-2. [DOI] [PubMed] [Google Scholar]
- Hoyer D. Molecular pharmacology and biology of 5-HT1C receptors. Trends Pharmacol Sci. 1988 Mar;9(3):89–94. doi: 10.1016/0165-6147(88)90174-5. [DOI] [PubMed] [Google Scholar]
- Hoyer D., Neijt H. C. Identification of serotonin 5-HT3 recognition sites in membranes of N1E-115 neuroblastoma cells by radioligand binding. Mol Pharmacol. 1988 Mar;33(3):303–309. [PubMed] [Google Scholar]
- Julius D., MacDermott A. B., Axel R., Jessell T. M. Molecular characterization of a functional cDNA encoding the serotonin 1c receptor. Science. 1988 Jul 29;241(4865):558–564. doi: 10.1126/science.3399891. [DOI] [PubMed] [Google Scholar]
- Kendall D. A., Nahorski S. R. 5-Hydroxytryptamine-stimulated inositol phospholipid hydrolysis in rat cerebral cortex slices: pharmacological characterization and effects of antidepressants. J Pharmacol Exp Ther. 1985 May;233(2):473–479. [PubMed] [Google Scholar]
- Kobilka B. K., Frielle T., Collins S., Yang-Feng T., Kobilka T. S., Francke U., Lefkowitz R. J., Caron M. G. An intronless gene encoding a potential member of the family of receptors coupled to guanine nucleotide regulatory proteins. Nature. 1987 Sep 3;329(6134):75–79. doi: 10.1038/329075a0. [DOI] [PubMed] [Google Scholar]
- Leysen J. E., Awouters F., Kennis L., Laduron P. M., Vandenberk J., Janssen P. A. Receptor binding profile of R 41 468, a novel antagonist at 5-HT2 receptors. Life Sci. 1981 Mar 2;28(9):1015–1022. doi: 10.1016/0024-3205(81)90747-5. [DOI] [PubMed] [Google Scholar]
- Leysen J. E., Niemegeers C. J., Van Nueten J. M., Laduron P. M. [3H]Ketanserin (R 41 468), a selective 3H-ligand for serotonin2 receptor binding sites. Binding properties, brain distribution, and functional role. Mol Pharmacol. 1982 Mar;21(2):301–314. [PubMed] [Google Scholar]
- Leysen J. E., de Chaffoy de Courcelles D., De Clerck F., Niemegeers C. J., Van Nueten J. M. Serotonin-S2 receptor binding sites and functional correlates. Neuropharmacology. 1984 Dec;23(12B):1493–1501. doi: 10.1016/0028-3908(84)90093-5. [DOI] [PubMed] [Google Scholar]
- Lübbert H., Hoffman B. J., Snutch T. P., van Dyke T., Levine A. J., Hartig P. R., Lester H. A., Davidson N. cDNA cloning of a serotonin 5-HT1C receptor by electrophysiological assays of mRNA-injected Xenopus oocytes. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4332–4336. doi: 10.1073/pnas.84.12.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lübbert H., Snutch T. P., Dascal N., Lester H. A., Davidson N. Rat brain 5-HT1C receptors are encoded by a 5-6 kbase mRNA size class and are functionally expressed in injected Xenopus oocytes. J Neurosci. 1987 Apr;7(4):1159–1165. doi: 10.1523/JNEUROSCI.07-04-01159.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh T. K., Barfield R. J., Geyer L. A. Ultrasonic vocalisations facilitate sexual behaviour of female rats. Nature. 1978 Mar 9;272(5649):163–164. doi: 10.1038/272163a0. [DOI] [PubMed] [Google Scholar]
- Nomura Y., Kaneko S., Kato K., Yamagishi S., Sugiyama H. Inositol phosphate formation and chloride current responses induced by acetylcholine and serotonin through GTP-binding proteins in Xenopus oocyte after injection of rat brain messenger RNA. Brain Res. 1987 Jul;388(2):113–123. doi: 10.1016/s0006-8993(87)80004-5. [DOI] [PubMed] [Google Scholar]
- Pazos A., Cortés R., Palacios J. M. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. II. Serotonin-2 receptors. Brain Res. 1985 Nov 4;346(2):231–249. doi: 10.1016/0006-8993(85)90857-1. [DOI] [PubMed] [Google Scholar]
- Peralta E. G., Ashkenazi A., Winslow J. W., Ramachandran J., Capon D. J. Differential regulation of PI hydrolysis and adenylyl cyclase by muscarinic receptor subtypes. Nature. 1988 Aug 4;334(6181):434–437. doi: 10.1038/334434a0. [DOI] [PubMed] [Google Scholar]
- Peroutka S. J. 5-Hydroxytryptamine receptor subtypes. Annu Rev Neurosci. 1988;11:45–60. doi: 10.1146/annurev.ne.11.030188.000401. [DOI] [PubMed] [Google Scholar]
- Peroutka S. J. Pharmacological differentiation and characterization of 5-HT1A, 5-HT1B, and 5-HT1C binding sites in rat frontal cortex. J Neurochem. 1986 Aug;47(2):529–540. doi: 10.1111/j.1471-4159.1986.tb04532.x. [DOI] [PubMed] [Google Scholar]
- Roth B. L., Nakaki T., Chuang D. M., Costa E. Aortic recognition sites for serotonin (5HT) are coupled to phospholipase C and modulate phosphatidylinositol turnover. Neuropharmacology. 1984 Oct;23(10):1223–1225. doi: 10.1016/0028-3908(84)90244-2. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Neher E., Sakmann B. Rat brain serotonin receptors in Xenopus oocytes are coupled by intracellular calcium to endogenous channels. Proc Natl Acad Sci U S A. 1987 Jul;84(14):5063–5067. doi: 10.1073/pnas.84.14.5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chaffoy de Courcelles D., Leysen J. E., De Clerck F., Van Belle H., Janssen P. A. Evidence that phospholipid turnover is the signal transducing system coupled to serotonin-S2 receptor sites. J Biol Chem. 1985 Jun 25;260(12):7603–7608. [PubMed] [Google Scholar]


