FIG 6.
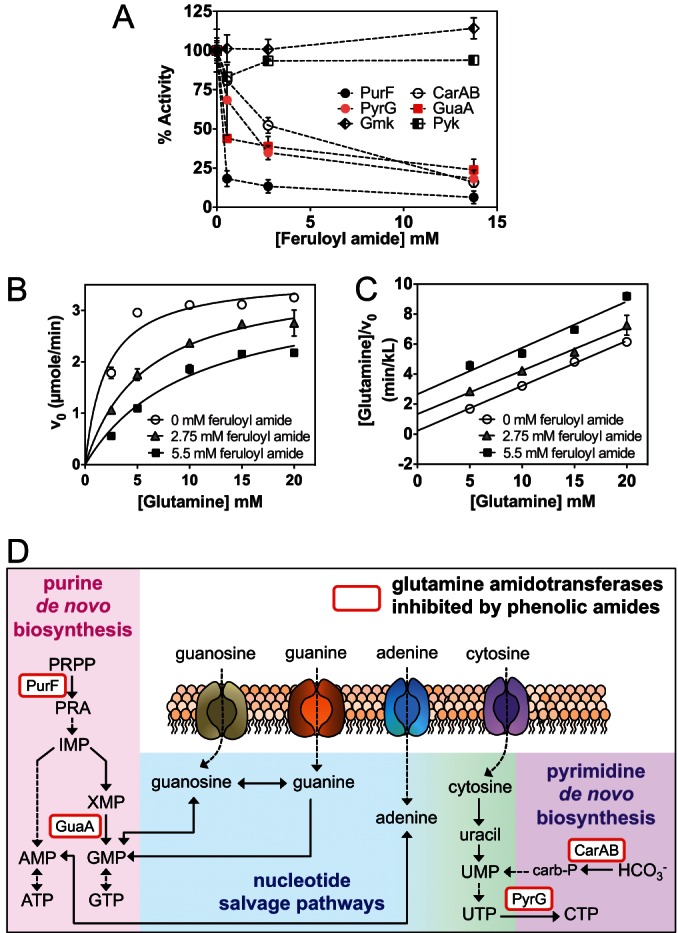
Phenolic amides inhibit glutamine amidotransferases in vitro. (A) Feruloyl amide inhibits glutamine amidotransferases in vitro. Enzymatic assay showed that feruloyl amide directly inhibits glutamine-PRPP-amidotransferase (PurF), GMP synthetase (GuaA), CTP synthetase (PyrG), and carbamoyl phosphate synthetase (CarA/CarB) but not guanylate kinase (Gmk) and pyruvate kinase (Pyk). The activity of each enzyme (measured as initial velocity) was normalized against that of the control. Data shown are an average of at least two technical replicates ± standard deviation (SD). (B and C) Feruloyl amide competitively inhibits PurF activity. (B) Averages of two biological replicates ± SD are fitted into a competitive inhibition equation, V0 = Vmax · [S]/(Km · (1 + [I]/Ki) + [S]). Data points in panel B were transformed into the Hanes-Woolf equation [S]/V0 = [S]/Vmax + Km/Vmax. (C) Parallel slopes in the Hanes-Woolf plot suggest competitive inhibitory mechanism. (D) Glutamine amidotransferases (rounded rectangles) directly inhibited by feruloyl amide and coumaroyl amide are involved in de novo nucleotide biosynthesis. Abbreviations: PRA, 5-phosphoribosylamine; IMP, inosine-5-monophosphate; XMP, xanthosine-5-monophosphate; carb-P, carbamoyl phosphate. Enzyme names: PurF, glutamine-PRPP-amidotransferase; GuaB, IMP dehydrogenase; GuaA, GMP synthetase; PyrG, CTP synthetase; CarAB, carbamoyl phosphate synthase.
