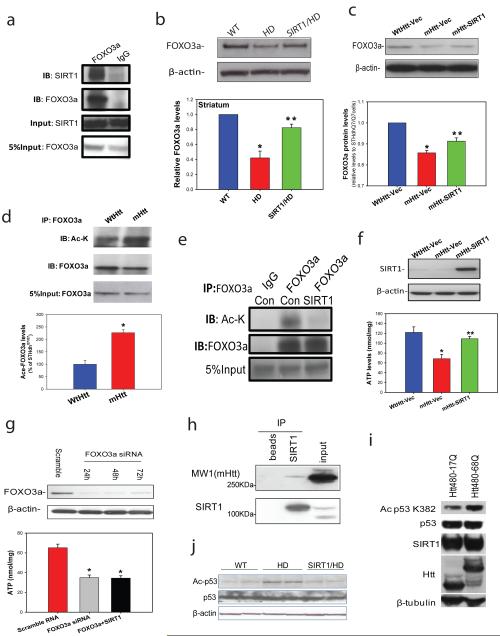
Figure 4. SIRT1 counteracts mutant Htt-induced hyperacetylation of FOXO3a and p53 and protects cells against mutant Htt-mediated energy deficits.
(a) Immunoprecipitation in mouse striatum indicates interactions between endogenous SIRT1 and FOXO3a. (b) FOXO3a levels were determined by Western blotting analysis in striatum of 14-week-old mice. Mean ± S.E.M., n=4. *p<0.05 vs wild type (WT) group; **p<0.05 vs HD group by Student’s t-tests. (c) FOXO3a levels were measured in cell lysates from striatal cells expressing wild type Htt (STHdhQ7/Q7, WtHtt) or mutant Htt (STHdhQ111/Q111, mHtt) transfected with vector (Vec), and mutant Htt expressing cells transfected with SIRT1(mHtt-SIRT1). Mean ± S.E.M. from three independent experiments. *p<0.05 vs WtHtt-Vec group; **p<0.05 vs mHtt-Vec group by Student’s t-tests. (d) Acetylated-FOXO3a levels are increased in cells expressing mutant Htt. Cell lysates were immunoprecipitated with FOXO3a antibody, and blotted with anti-acetylated lysine antibody. Upper panel shows representative blots and bottom panel shows the quantification data from three individual samples. *p<0.01 vs STHdh Q7/Q7 cells by Student’s t-tests. (e) SIRT1 deacetylates FOXO3a in striatal cells. STHdhQ111/Q111 cells were transfected with Vector (Con) or SIRT1 cDNAs for 24 h. Cell lysates were immunoprecipitated with FOXO3a antibody, and blotted with anti-acetylated lysine antibody. (f) SIRT1 protects striatal cells against mutant Htt-induced energy deficits. Striatal cells were cultured in DMEM for 24 h, SIRT1 was transfected into cells. Serum was removed 48 h after transfection, and ATP levels were measured at 24 h after serum withdrawal. The data are mean ± S.E.M. from three independent experiments. *p<0.05 vs the values of STHdhQ7/Q7 cells; **p<0.05 vs the values of vector-transfected STHdh Q111/Q111 cells by Student’s t-tests. (g) Knockdown of FOXO3a (top panel) enhanced mutant Htt-induced energy deficits and compromised the protective effects of SIRT1 (bottom panel). STHdhQ111/Q111 cells were transfected with FOXO3a siRNA (50 nM) or co-transfected with SIRT1, ATP levels were measured at 72 h after transfection. The data are mean ± S.E.M. from three independent experiments. *p<0.05 vs scrambled RNA transfected cells.*p<0.05 vs the values of scrambled RNA-treated cells by Student’s t-tests. (h) SIRT1 interacts with mutant huntingtin. Co-immunoprecipitation was performed with anti-SIRT1 antibody and probed for MW1 antibody which recognizes mutant huntingtin in mouse brain tissue. Beads were used to assess nonspecific binding. (i) Mutant Htt inhibits SIRT1 deacetylase activity which was assessed by p53 deacetylation at Lys 382. HEK T/17 cells transfected with SIRT1 and Htt480-17Q or Htt480-68Q were treated with 500 nM TSA and 100 µM etoposide to block class I and II HDAC activities and induce p53 expression. SIRT1 deacetylase activity was evaluated by monitoring endogenous p53 deacetylation at Lys 382. Equal expression of total p53, SIRT1, wild-type and mutant Htt was ensured by Western blotting with respective antibodies. β-Tubulin was used as loading control. Three independent experiments were performed and a representative blot is shown. (j) Acetyl-p53 and total p53 level were detected by Western blotting in cerebral cortex of 18-week-old mice of indicated genotypes.