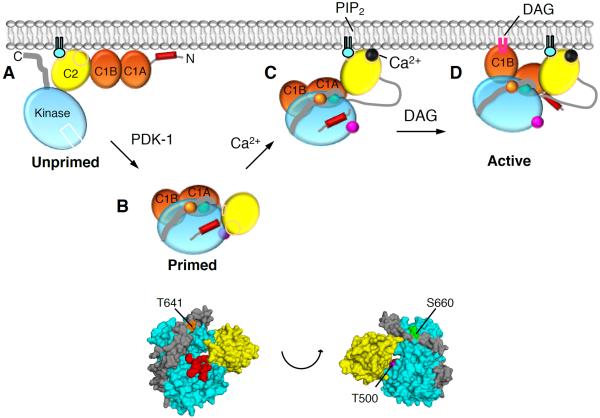
Figure 5. Model of PKCβII Activation.
(A) Unprimed PKCβII is in a membrane-associated, open conformation in which its C1A, C1B, and C2 domains are fully exposed and the pseudosubstrate and C-terminal tail are unmasked.
(B) Upon priming phosphorylation at its activation loop (T500, magenta) by PDK-1, followed by autophosphorylation at the turn motif (T641, orange) and the hydrophobic motif (S660, green), PKCβII matures into a closed conformation in which the C2 domain interfaces with the kinase domain and traps the pseudosubstrate into the substrate-binding site, both C1 domains become masked, and the primed enzyme localizes to the cytosol.
(C) In response to agonists that promote PIP2 hydrolysis, Ca2+ binds cytosolic PKCβII via a low affinity interaction such that upon the next diffusion-controlled membrane encounter, the Ca2+-bound C2 domain is retained at the plasma membrane via Ca2+-bridging to anionic lipids and binding to PIP2.
(D) Pre-targeted PKC binds the membrane-embedded ligand, DAG, predominantly via the C1B domain, resulting in release of the pseudosubstrate from the substrate-binding cavity, thereby activating PKC. Only one of the C1 domains binds DAG in the membrane at a time.