Figure 1.
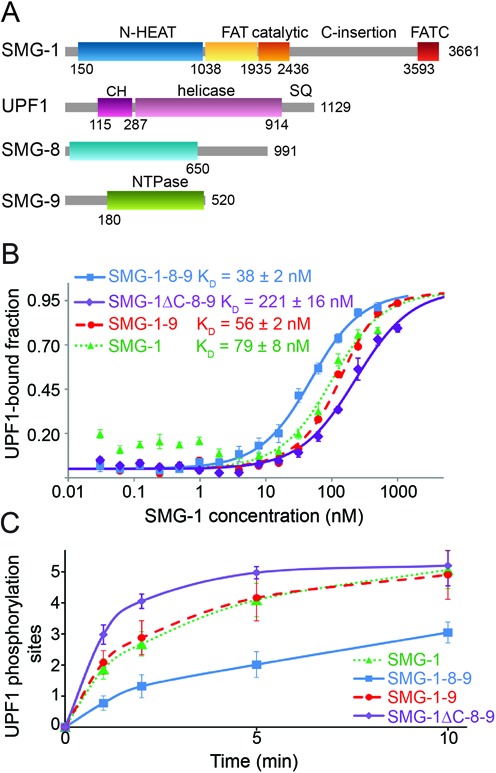
Binding and phosphorylation of UPF1 by SMG-1, SMG-1–9, SMG-1–8–9 and SMG-1ΔC-8–9. (A) Schematic representation of full-length SMG-1, UPF1, SMG-8 and SMG-9. Regions predicted to be structured are indicated with their domain boundaries. N-HEAT: N-terminal SMG-1 HEAT repeats, C-insertion: C-terminal insertion domain, CH: UPF1 cysteine-histidine-rich domain, SQ: UPF1 C-terminal unstructured region containing S/T-Q phosphorylation motifs. (B) Microscale thermophoresis experiments using fluorescently labeled UPF1 and increasing concentrations of SMG-1 (green), SMG-1–9 (red), SMG-1–8–9 (blue) or SMG-1ΔC-8–9 (violet). KD values from four independent experiments are indicated. (C) Kinetic in vitro UPF1 phosphorylation assays using SMG-1, SMG-1–9, SMG-1–8–9 and SMG-1ΔC-8–9. The number of phospho-sites in UPF1 was determined as a function of time in the presence of the same amount of SMG-1, SMG-1–9, SMG-1–8–9 or SMG-1ΔC-8–9, using ovalbumin as standard. Experiments were repeated at least three times.
