Figure 3.
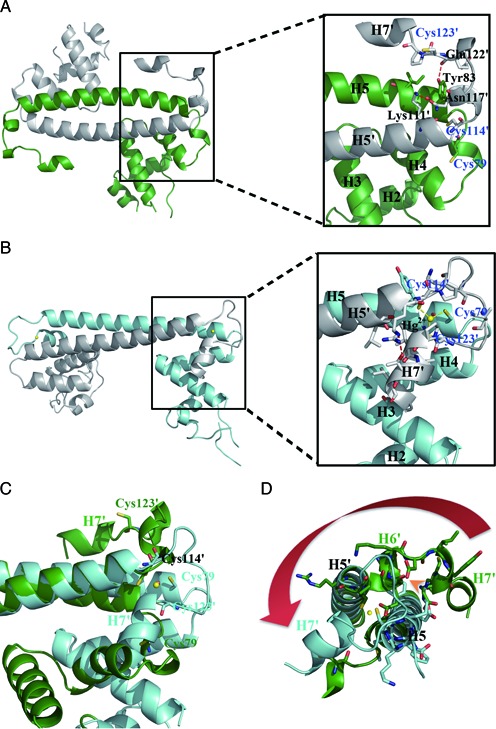
Detailed views of the Hg2+-induced movement of the metal-binding motif and assembly of the Hg2+ binding site. (A) Ribbon representation of apo-MerR homodimer (left) with an enlarged view (right) showing residues involved in the anchoring of the metal-binding motif as well as the spatial arrangement of the three Hg2+-ligating cysteine residues. (B) Ribbon representation of Hg2+-MerR homodimer (left) with an enlarged view (right) showing the coordination of Hg2+ (yellow sphere) by three cysteine residues and the involvement of H7 in Hg2+-binding. (C) Superimposition of apo-MerR (green) and Hg2+-MerR (cyan) shows that H7 and the three Hg2+-ligating cysteine residues undergo large repositioning upon Hg2+-binding. (D) The Hg2+-induced lengthening of H5 would result in steric clashes with the H5–H6 loop and H6 seen in apo-MerR structure (indicated by the orange arrowhead), causing conformational change in the metal-binding motif (highlighted by the red arrow). Labels belonging to the second protein subunit are flagged by a prime.
