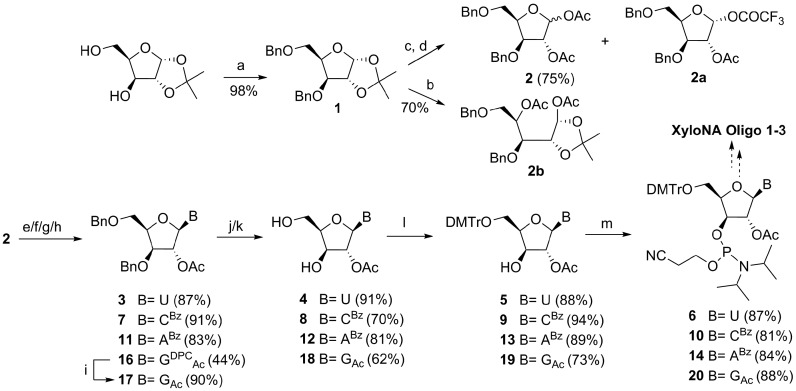
Scheme 1.
Schematic representation for the synthesis of four XyloNA nucleotide building blocks. Reagents and conditions: (a) BnBr, NaH, dry THF, 0°C–RT, 16 h; (b) AcOH, Ac2O, cat. H2SO4/CF3SO3H, 0°C–RT, 2 h; (c) 60% TFA in H2O, 0°C–RT, 4 h; (d) Ac2O, dry pyridine, 0°C–RT, 4 h; (e) for 3: uracil, BSA, CH3CN, 80°C, 1 h, then sugar 2, SnCl4, 50°C, 2 h; (f) for 7: N4-benzoylcytosine, BSA, CH3CN, 80°C, 45 min, then 2, SnCl4, 80°C, 1.5 h; (g) for 11: N6-benzoyladenine, 2, SnCl4, CH3CN, RT, 2 h; (h) for 16: N2-acetyl-O6-DPC guanine (15), BSA, DCE, 80°C, 30 min, then 2 in dry toluene, TMSOTf, 80°C, 1 h; (i) 90% TFA in H2O, RT, 2 h; (j) for 4: method A-Pd/C 10%, MeOH, H2, RT, 20 h or method B- BCl3, dry CH2Cl2, –78°C to –10°C, 3 h, then EtOH, –78/–40°C to RT, 30 min, 55%; for 8 and 12: method B, quenching with a mixture of EtOH and Et3N; (k) for 18: Pd/C 10%, Pd(OH)2/C 20%, H2O, MeOH, H2, RT, 72 h; (l) DMTr-Cl, dry pyridine, RT, 16 h; (m) NC(CH2)2OP(Cl)N(iPr)2, DIPEA, dry CH2Cl2, RT, 4 h.