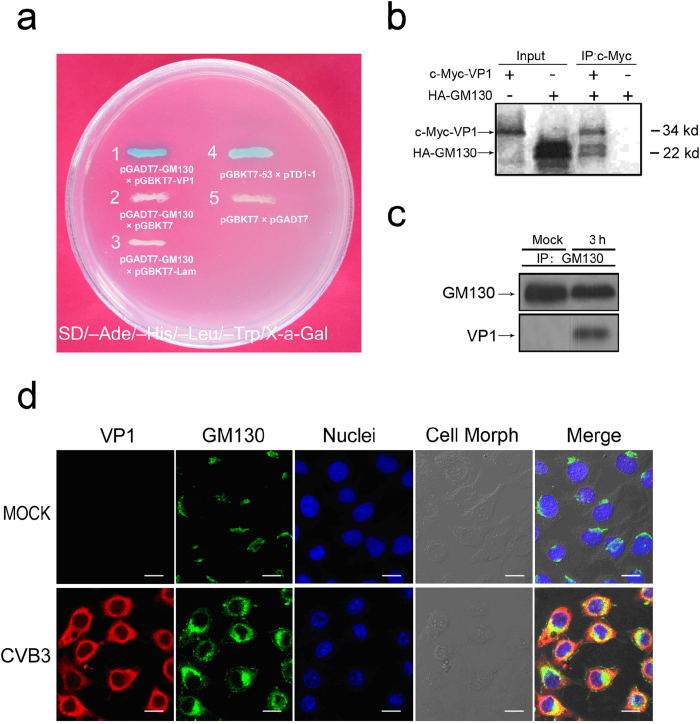
Figure 2. The interaction of CVB3 VP1 with human GM130.
(a) CVB3 VP1-human GM130 interaction in an Y2H system. Yeast reporter strain AH109 was cotransformed with pGADT7-GM130 and pGBKT7-VP1, or with pGADT7-GM130 and pGBKT7, or with pGADT7-GM130 and pGBKT7-Lam, or with pGBKT7-53 and pTD1-1 (positive control), or with pGBKT7 and pGADT7 (negative control). The transformed cells were analyzed for α-Galactosidase activity. (b) In vitro protein-protein binding assay between CVB3 VP1 and human GM130. Tagged proteins of c-Myc-VP1 and HA-GM130 (199 amino acids of C-terminal) were translated in vitro with [35S] methionine incorporation. After translation, c-Myc-VP1 and HA-GM130 (199 amino acids of C-terminal) were immunoprecipitated with monoclonal anti-c-Myc antibody, and the antibody–protein complexes were resolved in 10% SDS–PAGE and subjected to autoradiography. (c) CVB3 VP1 formed a complex with GM130 in vivo. Lysates of CVB3-infected HeLa cells were immunoprecipitated with monoclonal anti-GM130 antibody. Immune complexes (Lane 2), the GM130 control in normal HeLa cells (Lane 1), and the VP1 control in lysates from CVB3-infected HeLa cells (Lane 3) were detected by western blot analysis using anti-GM130 antibody or anti-VP1 antibody. (d) Colocalization of CVB3 VP1 and human GM130 under a FLUOVIEW FV1000 confocal laser scanning microscope (Olympus). CVB3-infected HeLa cells at 3 h p.i. were subjected to double immunofluorescent staining with antibodies against VP1 (red) or GM130 (green). Co-localization of VP1 and GM130 is indicated in yellow in the merged images. Nuclear staining with DAPI is shown in blue. Scale bar, 10 μm.