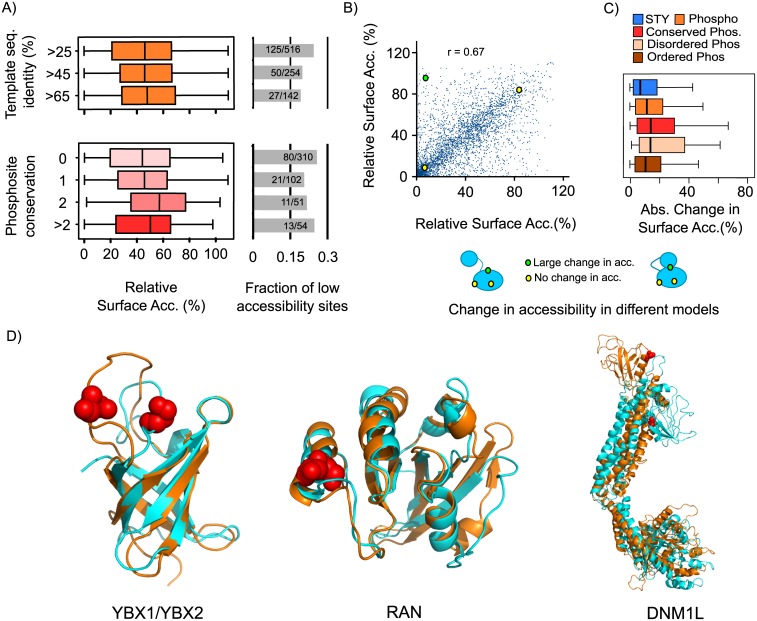
Fig 2. Phosphosites in solvent inaccessible positions may be predictive of conformational flexibility.
A) A small fraction of phosphosites (approximately 20%) was observed to be at solvent inaccessible positions (defined here as <20% all-atom RSA). The distribution of phosphosite RSA and the fraction of low accessibility sites do not vary significantly as a function of target-templates sequence identity nor phosphosite conservation. B) For phospho-acceptor residues (Serine, Threonine and Tyrosine) modeled independently based on more than one template structure, we compared the RSA values obtained from different models. These values are highly correlated, although some sites showed large variability in predicted accessibility, potentially indicating regions of conformational flexibility. C) We compared the changes in RSA in different models for phospho-acceptor residues, phosphoryation sites not known to be conserved, those conserved in at least 1 other species, phosphosites predicted by DISOPRED to be in ordered or disordered regions. D) Examples of phosphosites found in positions that show a large change in accessibility in two templates and are poorly accessibility in one of the templates. The phosphosite position is highlighted in red and the models in orange have a higher RSA for the phosphosite position when compared to the model in cyan.