Abstract
The appropriate exposure metrics for characterizing manganese (Mn) exposure associated with neurobehavioral effects have not been established. Blood levels of Mn (B-Mn) provide a potentially important intermediate marker of Mn airborne exposures. Using data from a study of a population of silicon- and ferro-manganese alloy production workers employed between 1973 and 1991, B-Mn levels were modeled in relation to prior Mn exposure using detailed work histories and estimated respirable Mn concentrations from air-sampling records. Despite wide variation in exposure levels estimated for individual jobs, duration of employment (exposure) was itself a strong predictor of B-Mn levels and strongest when an 80-day half-life was applied to contributions over time (t = 6.95, 7.44, respectively; p < 10 −5). Partitioning exposure concentrations based on process origin into two categories: (1) “large” respirable particulate (Mn-LRP) derived mainly from mechanically generated dust, and (2) “small” respirable particulate (Mn-SRP) primarily electric furnace condensation fume, revealed that B-Mn levels largely track the small, fume exposures. With a half-life of 65 days applied in a model with cumulative exposure terms for both Mn-LRP (t = −0.16, p = 0.87) and Mn-SRP (t = 6.45, p < 10 −5), the contribution of the large-size fraction contribution was negligible. Constructing metrics based on the square root of SRP exposure concentrations produced a better model fit (t = 7.87 vs. 7.44, R2 = 0.2333 vs. 0.2157). In a model containing both duration (t = 0.79, p = 0.43) and (square root) fume (t = 2.47, p = 0.01) metrics, the duration term was a weak contributor. Furnace-derived, small respirable Mn particulate appears to be the primary contributor to B-Mn levels, with a dose-rate dependence in a population chronically exposed to Mn, with air-concentrations declining in recent years. These observations may reflect the presence of homeostatic control of Mn levels in the blood and other body tissues and be useful in assessing Mn exposures for evaluating neurotoxic effects.
Keywords: Manganese, Respiratory particle size, Half-life, Blood manganese, Manganese alloy production
1. Introduction
It is well known that occupational exposure to manganese (Mn) is associated with neuropsychological and neurological deficits and disorders, on a continuum of severity, depending on external exposure dose (for review see: Mergler and Baldwin, 1997; Mergler et al., 1999; Zoni et al., 2007; Guillarte, 2010). Despite an extensive literature on this subject, results are inconsistent on the relations between exposure parameters and internal biomarkers of Mn (Smith et al., 2007). In general, Mn-exposed workers present higher blood Mn (B-Mn) compared to non-exposed referents (Mergler and Baldwin, 1997; Zheng et al., 2011), but few studies have observed dose-relations.
In their pioneering study of workers exposed to manganese oxides, Roels et al. (1987) reported that individual B-Mn concentration did not correlate with current exposures to Mn in total dust or to exposure duration; however, B-Mn was correlated to the chief foreman’s ranked estimation of integrated exposure for 11 departments. In an Italian study of ferroalloy workers, who were tested during a period of suspended production, B-Mn was positively correlated with an estimate of cumulative exposure to Mn in total dust (Lucchini et al., 1995). This workforce did not have Mn exposures 1–42 days prior to assessment. The authors hypothesize that B-Mn might reflect the rate at which excess manganese, accumulated over time, was being excreted. Interestingly, in a later follow-up of these same workers but now with current Mn exposures, there was a significant positive correlation between B-Mn and airborne Mn in total dust, but no association with estimated cumulative exposure (Lucchini et al., 1999). In a Norwegian study of Mn alloy production workers, where both inhalable and respirable Mn were measured, B-Mn showed a weak association with the respirable fraction, but no association with inhalable (Ellingsen et al., 2003). A South African study of smelter workers showed a positive correlation between B-Mn and Mn intensity in current job, and increasing B-Mn with time spent in that particular job, but not with overall employment duration (Myers et al., 2003). In a study of bridge welders working in confined spaces for 2 years or less, B-Mn was predicted by both duration and cumulative exposure but prediction improved when a half-life of 150 days was applied to Mn exposure levels (Park et al., 2009).
Systemic uptake of Mn occurs largely via the pulmonary route because excess Mn absorbed from the GI tract is efficiently eliminated (Andersen et al., 1999). Larger inhaled particles (>10.0 µm) are generally transported to the GI tract. Uptake of very small (nano-sized) particles containing Mn via the olfactory nerve has been proposed (Elder et al., 2006; Sunderman, 2001). The relative potency of airborne Mn in the respirable size range, when present as larger (dust) or smaller (fume) particulate, remains to be determined.
Manganese alloy production workers are exposed to a wide range of particulate size. In 1994, Mergler et al. (1994) published a matched pair study of neurobehavioral outcomes, comparing Mn alloy workers to a referent group of non-occupationally exposed workers from the same geographic region. The plant in Quebec, Canada, was in operation from 1973 to 1991 and, at the time of the study, the geometric mean for Mn in total dust was 0.23 mg/m3 and in the respirable fraction was 0.04 mg/m3 (Mergler et al., 1994). Baldwin et al. (2008) developed exposure histories for the workers from this plant for use in follow-up studies (Bouchard et al., 2007a,b, 2008).
The initial goal of the current investigation was to use this extensive database to examine the relations between B-Mn and history of respirable Mn exposure, with a view to determining the optimum exposure metric for predicting B-Mn. Since preliminary analyses indicated that the duration of exposure (with half-life weighting) was itself an important predictor of B-Mn levels, we decided to distinguish respirable airborne Mn in dust of mechanical origin, thought to be relatively large respirable particulate (Mn-LRP), from condensation fume assumed to be relatively small respirable particulate (Mn-SRP). The latter was assumed to have slower sedimentation rates and be more uniformly distributed across the alloy facility due to its much smaller particle size. In the absence of detailed particle-size information, we assume that Mn-LRP exposures were generally >1.0 µm in mass median aerodynamic diameter and Mn-SRP exposures <1.0 µm diameter. The present study investigates the separate contributions of Mn-LRP and Mn-SRP to B-Mn concentrations in these Mn alloy production workers.
2. Methods
2.1. Database
The original study recruited 115 workers from a ferro- and silico-Mn alloy production plant (95% of the total workforce) and 145 non-exposed workers from the same community as referents (Mergler et al., 1994). All production was in batches using a single, sealed submerged electric arc furnace. The retrospective Mn exposure assessment is published elsewhere (Baldwin et al., 2008). It was based on work history and environmental sampling. Work history consisted of the sequence of job group assignments from payroll records for each worker with associated dates. Individual exposure profiles of many workers were complex due to frequent job rotations with differing exposure levels. Some workers held more than 20 job assignments. During plant operations from 1973 to 1991, there was relatively little work-force turnover. At the time of the neurobehavioral assessment, during the fourth quarter of 1990, blood samples were taken prior to exposure on the last day of the worker’s shift. Methods of blood sampling and Mn determinations by flameless atomic absorption spectrophotometry are described in Mergler et al. (1994).
Exposure measurements included: (a) results from a 1990– 1991 industrial hygiene survey, which provided full-shift personal and area sampling data for particulate, not otherwise specified (PNOS) (i.e., total gravimetric dust) and for Mn content in the dust, together with full-shift area samples of respirable dust and its Mn content; (b) historical short-term personal and area total dust samples from 1978 to 1984; and (c) three surveys of the furnace team between 1987 and 1989, which contained personal air sampling data for total dust and Mn content (Baldwin et al., 2008). The compilation of historical exposure information, additional air sampling in 1990–1991, and the construction of retrospective exposure estimates of respirable Mn were performed by professional industrial hygienists under the supervision of one of the study investigators (M. Baldwin, CIH, ROH (Canada)).
In the exposure reconstruction (Baldwin et al., 2008), which is briefly summarized here, job groups were defined. Estimates for total manganese were based on averages within fourteen discrete time periods, and changes between periods were derived from documented work practice and/or ventilation changes, or plant closures. Because there were no data earlier than 1978, and because no major modifications (other than plant closures) had taken place between 1973 and 1978, the assumption was made that conditions in 1978 were representative of those prior to that time. In the absence of personal respirable sampling data, estimates of respirable manganese for the job groups were based on the paired area sampling data collected in April–March of 1991.
Most jobs sampled in 1991 were located in the four main areas of the plant: the raw materials yard, the furnace floor, the crushing/end product handling area and the maintenance building. The ratio of the geometric mean (GM) of respirable Mn to total Mn for a given area was applied to all total Mn estimates over time for jobs within that area. This assumes that the ratio of respirable to total Mn was characteristic of a given area and stable over time and that the area ratio was applicable to personal exposure for job groups located in that area. For those who worked across the plant, the overall ratio for the plant was used. For the few jobs in the sinter plant, which closed in 1988, 5% of total Mn was stipulated to be respirable, as the very heavy dust levels in this area were mechanically derived from slag and coke. Those exposures would have made a small contribution to blood levels 3 years later.
2.2. Construction of dust and fume exposure metrics
To investigate the separate contributions of Mn-LRP and Mn-SRP to Mn in total respirable particulate (Mn-TRP), corresponding exposure metrics were constructed (Table 1). Using the job and process descriptions observed by Baldwin et al. (2008), the general exposure status of all jobs was classified in four groups:
Primarily fume: In these jobs, the men worked in close proximity to furnace operations on the furnace floor, with exposure to fumes from the hot metal pours and no major mechanical dust source. The estimate for Mn-TRP is based on the data reported for the furnace team, was entirely allocated to Mn-SRP exposure; Mn-LRP was set to zero.
Both dust and fume: The jobs assigned to this category had substantial exposure to both mechanical dust and fume. Included were those in the product crushing area, which was in close proximity to the furnace bays, and maintenance workers, who worked in the furnace area. They were assigned the Mn-SRP of the furnace team and the Mn-LRP value was set as the difference between Mn-TRP for the job and the assigned Mn-SRP value. If the assigned Mn-SRP value was greater than half of the Mn-TRP for a job, then both Mn-LRP and Mn-SRP were set as half the Mn-TRP.
Primarily dust: These jobs involved mechanical crushing and handling more distant from furnace fume generation. They mainly included jobs in the raw materials yard and the sinter operation. They were assigned the intermediate Mn-SRP level of the furnace loader operator, which was lower than the furnace team; Mn-LRP was calculated as above.
Ambient: These jobs were distant from the furnace and Mn crushing operations, e.g. in the maintenance workshop, or in the silicon plant, and the Mn-SRP concentration was set to the Mn-TRP for the job and Mn-LRP as set to zero.
Table 1.
Job group exposure assignment procedure.
| Job group | General exposure status |
% Resp | Fume-analog job group | Procedure |
|---|---|---|---|---|
| Furnace team | Primarily Fume | 13 | Assign fume concentration based on estimated respirable | |
| Furnace, laborer | 13 | Mn for Job. | ||
| Furnace loader opr | 13 | Assign dust concentration=0 | ||
| Furnace, sampler | 13 | |||
| Instrument. tech | 13 | |||
| Furnace, gas system | 13 | |||
| Furnace, Kress opr | 13 | |||
| Control rm opr | Dust and Fume | 19 | Furnace team | Assign fume concentration based on estimated respirable |
| Product crushing, other | 12 | Furnace team | Mn in Fume-analog Job. | |
| Electricians | 12 | Furnace team | Derive dust concentration as difference between estimated respirable Mn for Job minus assigned fume concentration. If assigned fume conc. >(estimated respirable Mn for Job)/2, then set both dust and fume=(estimated respirable Mn for Job)/2 |
|
| Welders | 12 | Furnace team | ||
| Fitters | 12 | Furnace team | ||
| Product crushing, hopper | 12 | Furnace team | ||
| Equip opr, raw matl | Primarily Dust | 22 | Furnace loader opr | |
| Yard laborer | 22 | Furnace loader opr | ||
| Janitor | 19 | General equip. maint., head | ||
| Sinter plant, opr | 5 | Furnace loader opr | ||
| Sinter plant, laborer, asst opr | 5 | Furnace loader opr | ||
| Sinter plant, foreman | 5 | Furnace loader opr | ||
| Product crushing, brakeman | 12 | Furnace loader opr | ||
| Maintenance, dust collector | 11 | Furnace loader opr | ||
| Yard, truck/machine | 11 | Furnace loader opr | ||
| Maintenance shop | Ambient | 21 | Assign fume concentration based on estimated respirable | |
| Silicon plant | 10 | Mn for Job. | ||
| General equip. maint., head | 21 | Assign dust concentration=0 | ||
| Maintenance, supvr | 21 |
Fume analog: similar job group with only fume exposure; used to assign fume level to jobs with dust exposure.
For each job, in each exposure period, the resulting estimates for Mn-LRP and Mn-SRP concentrations summed to the estimate for Mn-TRP derived from the air sampling data. The choices made in this classification were based on observation of plant operations and physical configuration by the industrial hygienist-investigator, as well as the area air sampling results. With the exception of a revised adjustment in some levels accounting for the closure of the sintering operations, a major dust contributor, these specifications were defined prior to detailed analyses for predicting B-Mn, but after the observation that exposure duration by itself was a major predictor of B-Mn.
2.3. Exposure metrics
To model blood manganese levels, cumulative respirable Mn exposure was calculated in the usual manner as a time-weighted sum of job assignment exposures up until the date of blood collection. Because excess Mn is cleared from the body, Mn exposure burdens (BMn) were calculated, as follows:
where X(i) is the estimated Mn-TRP (Mn-LRP or Mn-SRP) at time, i; t1 is the index of time periods corresponding to the first exposure, t2 corresponds to the last exposure and T½ is the half-life or time-constant for the declining contribution of an Mn exposure to future blood levels. Time, i, in this calculation, was partitioned in 10-day units. With half-life approaching ∞, BMn becomes the usual cumulative exposure. The range of half-lives analyzed was based on physiological plausibility, recognizing that complex clearance patterns from diverse tissues are present and being summarized with a single constant. When applied to Mn exposures this time-weighting was also applied to duration of Mn exposure. In order to assess dose-rate effects, where the air concentration at a point in time does not contribute proportionately to the cumulative exposure or burden metrics, the metrics were also calculated based on the square root and square of the estimated air Mn concentrations.
2.4. Statistical analysis
Blood Mn was modeled in relation to past Mn exposure, age and education. Multiple linear regression models were fit using proc REG in SAS (SAS Institute, 2011). Regression diagnostics were examined. Age centered at 40 yr, and education centered at 12 yr, together with their squares, were included in models whether or not statistically significant, to adjust for possible physiological effects (age) and possible exposure misclassification related to skill status (education). These effects were very small. Different exposure metrics were compared based on model R2 and exposure-term associated t-statistics. The model form was as follows:
where metX is an exposure metric: duration, cumX, etc.
3. Results
Study population: Following exclusion for missing data on B-Mn, education or age, 104 alloy production workers and 131 non-exposed workers from the surrounding region were used in the models. The average age of the workers and the referents was 44.2 ± 5.8 and 43.1 ± 7.2 years, respectively, and average years of education was 10.6 ± 2.1 and 10.8 ± 2.6, respectively. The mean employment duration of the Mn alloy workers was 14.4 yr ± 1.8, ranging from 7.4 to 16.1 yr.
3.1. Exposure matrix
Based on the process described in Table 1, Tables 2 and 3 present the mean Mn-LRP and the Mn-SRP, respectively, for the different job groupings over selected periods. For the most part, respirable Mn concentrations in dust and fume declined after 1987. Mn-LRP concentrations were higher than Mn-SRP in the early years, but not after closing of sinter operations in 1988. In the study plant population at the time of the survey, the mean time-weighted average exposure of each worker was for Mn-LRP: 0.065 mg/m3, for Mn-SRP: 0.082 mg/m3 and for Mn-TRP: 0.148 mg/m3. The mean cumulative exposures were Mn-LRP: 0.918 mg/m3-yr; Mn-SRP: 1.175 mg/m3-yr; Mn-TRP: 2.093 mg/m3-yr.
Table 2.
Estimated large respirable particulate exposure: mg/m3 as respirable Mn (Mn-LRP) in selected time periods
| Period | 1 | 3 | 5 | 6 | 8 | 9 | 10 | 11 | 14 |
|---|---|---|---|---|---|---|---|---|---|
| Mid-year | 1974 | 1977 | 1979 | 1982 | 1985 | 1987 | 1988 | 1989 | 1990 |
| Primarily fume | |||||||||
| Furnace team | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Furnace, laborer | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Furnace loader opr | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Furnace, sampler | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Instrument. tech | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Furnace, gas system | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Furnace, Kress opr | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Dust and fume | |||||||||
| Control rm opr | 0.011 | 0.011 | 0.006 | 0.011 | 0.037 | 0.008 | 0.008 | 0.006 | 0.003 |
| Product crushing, other | 0.087 | 0.087 | 0.092 | 0.087 | 0.055 | 0.055 | 0.055 | 0.055 | 0.023 |
| Electricians | 0.386 | 0.386 | 0.470 | 0.386 | 0.276 | 0.433 | 0.148 | 0.099 | 0.044 |
| Welders | 0.086 | 0.086 | 0.090 | 0.086 | 0.086 | 0.086 | 0.040 | 0.026 | 0.012 |
| Fitters | 0.269 | 0.269 | 0.353 | 0.269 | 0.217 | 0.316 | 0.105 | 0.070 | 0.031 |
| Product crushing, hopper | 0.169 | 0.169 | 0.252 | 0.169 | 0.167 | 0.215 | 0.215 | 0.254 | 0.000 |
| Primarily dust | |||||||||
| Equip opr, raw matl | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 | 0.003 | 0.003 | 0.003 |
| Yard laborer | 0.031 | 0.031 | 0.031 | 0.031 | 0.031 | 0.032 | 0.004 | 0.004 | 0.004 |
| Janitor | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 |
| Sinter plant, opr | 0.030 | 0.030 | 0.030 | 0.030 | 0.030 | 0.030 | 0.000 | 0.000 | 0.000 |
| Sinter plant, laborer, asst opr | 0.458 | 0.458 | 0.458 | 0.458 | 0.458 | 0.475 | 0.000 | 0.000 | 0.000 |
| Sinter plant, foreman | 0.138 | 0.138 | 0.138 | 0.138 | 0.138 | 0.155 | 0.000 | 0.000 | 0.000 |
| Product crushing, brakeman | 0.097 | 0.097 | 0.097 | 0.097 | 0.097 | 0.114 | 0.114 | 0.121 | 0.000 |
| Maintenance, dust collector | 0.288 | 0.288 | 0.288 | 0.288 | 0.281 | 0.181 | 0.181 | 0.142 | 0.032 |
| Yard, truck/machine | 0.020 | 0.020 | 0.020 | 0.020 | 0.020 | 0.013 | 0.013 | 0.010 | 0.007 |
| Ambient | |||||||||
| Maintenance shop | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Silicon plant | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| General equip. maint., head | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Maintenance, supvr | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
Derived from estimates of respirable Mn (arithmetic means) (Baldwin et al., 2008). Displayed for 9 of 14 time periods that included Periods 2 (Jan. 1976–June 1976) and 4 (Jan. 1978–June 1979) when the plant was closed.
Table 3.
Estimated small respirable particulate exposure: mg/m3 as respirable Mn (Mn-SRP) in selected time periods.
| Period | 1 | 3 | 5 | 6 | 8 | 9 | 10 | 11 | 14 |
|---|---|---|---|---|---|---|---|---|---|
| Mid-year | 1974 | 1977 | 1979 | 1982 | 1985 | 1987 | 1988 | 1989 | 1990 |
| Primarily fume | |||||||||
| Furnace team | 0.165 | 0.165 | 0.082 | 0.165 | 0.528 | 0.118 | 0.118 | 0.079 | 0.035 |
| Furnace, laborer | 0.395 | 0.395 | 0.395 | 0.395 | 0.387 | 0.250 | 0.250 | 0.195 | 0.057 |
| Furnace loader opr | 0.047 | 0.047 | 0.047 | 0.047 | 0.047 | 0.030 | 0.030 | 0.023 | 0.017 |
| Furnace, sampler | 0.135 | 0.135 | 0.135 | 0.135 | 0.135 | 0.118 | 0.118 | 0.079 | 0.035 |
| Instrument. tech | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 | 0.008 |
| Furnace, gas system | 0.165 | 0.165 | 0.082 | 0.165 | 0.165 | 0.118 | 0.118 | 0.079 | 0.035 |
| Furnace, Kress opr | 0.047 | 0.047 | 0.047 | 0.047 | 0.047 | 0.030 | 0.030 | 0.023 | 0.017 |
| Dust and fume | |||||||||
| Control rm opr | 0.011 | 0.011 | 0.006 | 0.011 | 0.037 | 0.008 | 0.008 | 0.006 | 0.003 |
| Product crushing, other | 0.087 | 0.087 | 0.082 | 0.087 | 0.055 | 0.055 | 0.055 | 0.055 | 0.023 |
| Electricians | 0.165 | 0.165 | 0.082 | 0.165 | 0.276 | 0.118 | 0.118 | 0.079 | 0.035 |
| Welders | 0.086 | 0.086 | 0.082 | 0.086 | 0.086 | 0.086 | 0.040 | 0.026 | 0.012 |
| Fitters | 0.165 | 0.165 | 0.082 | 0.165 | 0.217 | 0.118 | 0.105 | 0.070 | 0.031 |
| Product crushing, hopper | 0.165 | 0.165 | 0.082 | 0.165 | 0.167 | 0.118 | 0.118 | 0.079 | 0.000 |
| Primarily dust | |||||||||
| Equip opr, raw matl | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 | 0.010 | 0.003 | 0.003 | 0.003 |
| Yard laborer | 0.031 | 0.031 | 0.031 | 0.031 | 0.031 | 0.030 | 0.004 | 0.004 | 0.004 |
| Janitor | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 |
| Sinter plant, opr | 0.030 | 0.030 | 0.030 | 0.030 | 0.030 | 0.030 | 0.000 | 0.000 | 0.000 |
| Sinter plant, laborer, asst opr | 0.047 | 0.047 | 0.047 | 0.047 | 0.047 | 0.030 | 0.000 | 0.000 | 0.000 |
| Sinter plant, foreman | 0.047 | 0.047 | 0.047 | 0.047 | 0.047 | 0.030 | 0.000 | 0.000 | 0.000 |
| Product crushing, brakeman | 0.047 | 0.047 | 0.047 | 0.047 | 0.047 | 0.030 | 0.030 | 0.023 | 0.000 |
| Maintenance, dust collector | 0.047 | 0.047 | 0.047 | 0.047 | 0.047 | 0.030 | 0.030 | 0.023 | 0.017 |
| Yard, truck/machine | 0.020 | 0.020 | 0.020 | 0.020 | 0.020 | 0.013 | 0.013 | 0.010 | 0.007 |
| Ambient | |||||||||
| Maintenance shop | 0.020 | 0.020 | 0.020 | 0.020 | 0.020 | 0.020 | 0.020 | 0.020 | 0.020 |
| Silicon plant | 0.001 | 0.001 | 0.001 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| General equip. maint., head | 0.020 | 0.020 | 0.020 | 0.020 | 0.020 | 0.020 | 0.020 | 0.020 | 0.020 |
| Maintenance, supvr | 0.020 | 0.020 | 0.020 | 0.020 | 0.020 | 0.020 | 0.020 | 0.020 | 0.020 |
Derived from estimates of respirable Mn (arithmetic means) (Baldwin et al., 2008). Displayed for 9 of 14 time periods that included Periods 2(Jan. 1976–June 1976) and 4 (Jan. 1978–June 1979) when the plant was closed.
3.2. Models of blood manganese levels
In analyses controlling for age and education, workers ever employed in the Mn plant compared to the referent group had a highly statistically significant elevation of manganese in blood (B-Mn) (t = 7.14, R2 = 0.2032, p < 10−10) (Table 4). The model-predicted value of B-Mn in the comparison group was 7.2 µg/L and in the exposed group was 10.64 µg/L (data not shown). Duration of Mn exposure was a comparable predictor of B-Mn but with slightly inferior fit (R2 = 0.1956) (Table 4). The usual Mn cumulative exposure metrics, for Mn-TRP, Mn-LRP and Mn-SRP, were weaker predictors of B-Mn than duration, particularly Mn-LRP (Table 4).
Table 4.
Blood manganese (B-Mn) models with Mn duration and Mn respirable particulate cumulative exposures.
| Exposure metric | R2 | t | p |
|---|---|---|---|
| None | 0.0253 | – | |
| Ever exposed (0.1) | 0.2032 | 7.14 | <10−10 |
| Duration of exposure, yr | 0.1956 | 6.95 | <10−10 |
| Cumulative exposure: total respirable Mn (Mn-TRP) | 0.1345 | 5.37 | 8 × 10−8 |
| Cumulative exposure: large particulate respirable Mn (Mn-LRP) | 0.0885 | 3.98 | 7 × 10−5 |
| Cumulative exposure: small particulate respirable Mn (Mn-SRP) | 0.1516 | 5.83 | 6 × 10−9 |
With exclusions for missing data on B-Mn, education or age, n: 104 Mn-alloy production workers, 131 non-exposed workers. Model: B-Mn = α + β(education-12) + γ(education-12)2+ δ(age-40) + ε(age-40)2 +η(metX), where metX is an exposure metric: duration, cumX, etc.
Table 5 presents a series of models for B-Mn using different measures of exposure at increasing Mn half-life. Exposure metrics in the form of duration with a half-life of 80 days or burdens with a half-life in the range 60–65 days better predicted B-Mn (Table 5) than metrics without an applied half-life. Mn-SRP burden (half-life = 60 days) was the best predictor (t = 7.46, R2 = 0.216, model 12) but only slightly better than duration (t = 7.41, R2 =0.215, model 9) and Mn-LRP was the poorest predictor (t = 3.40, R2 = 0.072, model 11). Terms for education and age were statistically insignificant except for a suggestion of a negative effect for the linear education term.
Table 5.
Blood manganese (B-Mn) models based on respirable Mn exposure duration and particulate exposure variables with applied half-life, and dose rate effect.
| Model | Exposure metric | Linear dose-ratea |
Square root dose-rateb |
||
|---|---|---|---|---|---|
| R2 | t | R2 | t | ||
| Duration/Mn half-life: 40 days | |||||
| 1 | Duration of exposure | 0.2122 | 7.36 | ||
| 2 | Total respirable (Mn-TRP) | 0.1712 | 6.34 | ||
| 3 | Large particulate (Mn-LRP) | 0.0712 | 3.36 | ||
| 4 | Small particulate (Mn-SRP). | 0.2147 | 7.42 | ||
| Duration/Mn half-life: 50 days | |||||
| 5 | Duration of exposure | 0.2136 | 7.39 | ||
| 6 | Total respirable (Mn-TRP) | 0.1726 | 6.37 | ||
| 7 | Large particulate (Mn-LRP) | 0.0719 | 3.39 | ||
| 8 | Small particulate (Mn-SRP) | 0.2162 | 7.45 | ||
| Duration/Mn half-life: 60 days | |||||
| 9 | Duration of exposure | 0.2145 | 7.41 | 0.2145 | 7.41 |
| 10 | Total respirable (Mn-TRP) | 0.1736 | 6.40 | 0.2200 | 7.54 |
| 11 | Large particulate (Mn-LRP) | 0.0724 | 3.40 | 0.0903 | 4.04 |
| 12 | Small particulate (Mn-SRP) | 0.2164 | 7.46 | 0.2333 | 7.86 |
| Duration/Mn half-life: 65 days | |||||
| 13 | Duration of exposure | 0.2148 | 7.42 | 0.2148 | 7.42 |
| 14 | Total respirable (Mn-TRP) | 0.1739 | 6.40 | 0.2202 | 7.55 |
| 15 | Large particulate (Mn-LRP) | 0.0726 | 3.41 | 0.0906 | 4.05 |
| 16 | Small particulate (Mn-SRP) | 0.2162 | 7.45 | 0.2334 | 7.87 |
| Duration/Mn half-life: 70 days | |||||
| 17 | Duration of exposure | 0.2151 | 7.43 | 0.2151 | 7.43 |
| 18 | Total respirable | 0.1740 | 6.41 | 0.2203 | 7.55 |
| 19 | Large particulate (Mn-LRP) | 0.0727 | 3.42 | 0.0909 | 4.06 |
| 20 | Small particulate (Mn-SRP) | 0.2157 | 7.44 | 0.2333 | 7.87 |
| Duration/Mn half-life: 75 days | |||||
| 21 | Duration of exposure | 0.2154 | 7.43 | 0.2154 | 7.43 |
| 22 | Total respirable (Mn-TRP) | 0.1741 | 6.41 | 0.2203 | 7.55 |
| 23 | Large particulate (Mn-LRP) | 0.0728 | 3.42 | 0.0911 | 4.06 |
| 24 | Small particulate (Mn-SRP) | 0.2151 | 7.43 | 0.2332 | 7.86 |
| Duration/Mn half-life: 80 days | |||||
| 25 | Duration of exposure | 0.2155 | 7.44 | ||
| 26 | Total respirable (Mn-TRP) | 0.1740 | 6.41 | ||
| 27 | Large particulate (Mn-LRP) | 0.0729 | 3.42 | ||
| 28 | Small particulate (Mn-SRP) | 0.2144 | 7.41 | ||
| Duration/Mn half-life: 240 days | |||||
| 29 | Duration of exposure | 0.2145 | 7.41 | ||
| 30 | Total respirable (Mn-TRP) | 0.1510 | 5.81 | ||
| 31 | Large particulate (Mn-LRP) | 0.0689 | 3.27 | ||
| 32 | Small particulate (Mn-SRP) | 0.1786 | 6.52 | ||
| Duration/Mn half-life: 960 days | |||||
| 33 | Duration of exposure | 0.2074 | 7.24 | ||
| 34 | Total respirable (Mn-TRP) | 0.1269 | 5.15 | ||
| 35 | Large particulate (Mn-LRP) | 0.0724 | 3.41 | ||
| 36 | Small particulate (Mn-SRP) | 0.1340 | 5.35 | ||
Separate model for each exposure metric/half-life/dose-rate. With exclusions for missing data on B-Mn, education, age or work history, n: 103 Mn-alloy production workers, 131 non-exposed workers. Model: B-Mn = α + β(education-12)+γ(education-12)2+ δ(age-40) + ε(age-40)2 +η (metX), where metX is an exposure metric: duration, cumX, etc.
Burden calculated with linear intensity dependence.
Burden calculated with square-root of intensity dependence.
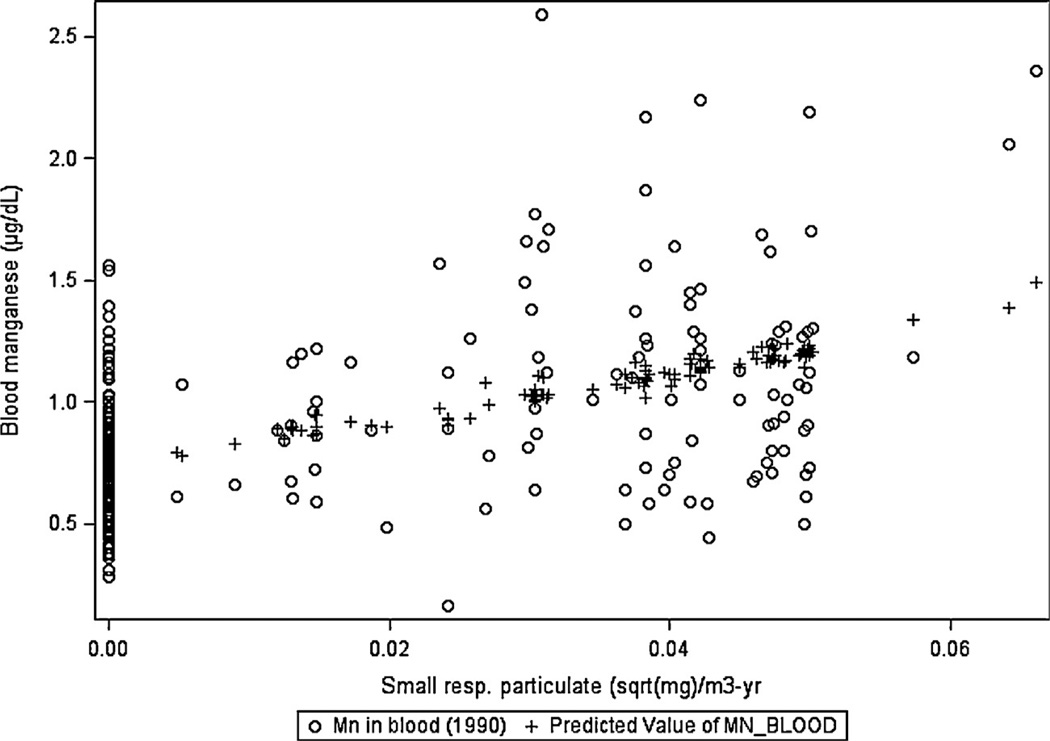
Burdens were also calculated based on the square root or square transformation of Mn exposure metrics to address possible dose-rate effects. The burden using square root of Mn-SRP exposure with a half-life 65 days produced a model fitting somewhat better (t = 7.87, R2 = 0.233) than with duration or untransformed Mn-SRP (Table 5; models 13 and 16). Fig. 1 displays the observed and predicted values of B-Mn with square root of Mn-SRP exposure; the comparison population (exposure = 0) is included. Mn-TRP (model 14) and Mn-LRP (model 15) also improved when based on the square root of Mn concentration. Burdens based on the square of exposure intensity were poor predictors of B-Mn (data not shown). Short half-lives of 1–4 days, corresponding to the known rapid elimination of recent Mn exposure, produced poor fitting models, and when included along with the best predictor (square root of cumulative Mn fume, 65 day half-life) did not at all improve model fit (data not shown).
Fig. 1.
Blood manganese: actual vs. predicted by small resp. particulate (cum. sq. root) for both alloy workers and community worker controls corresponding to Table 5, model 16 and Table 6, model 6.
Table 6 presents models of B-Mn with competing Mn exposure metrics, using a half-life of 65-days. Models with the best Mn Mn-SRP metric, together with duration or Mn-LRP terms, demonstrate the dominance of Mn-SRP in predicting blood Mn levels. Using both Mn-LRP and Mn-SRP, with no dose-rate effect (model 4), the Mn-LRP contribution is insignificant (t = 0.16) and negative; with duration and Mn-SRP (model 5) both terms are important predictors of comparable fit (t = 2.45, 2.53, respectively). Allowing for the dose-rate effect using square root of exposure intensity in calculating burden, the unimportance of Mn-LRP was again observed (model 7) and now Mn-SRP (t = 2.47) was a much better predictor when competing with duration (t = 0.79) (model 8). In the models presented in Table 6, regression diagnostics revealed no important departures in distributions of residuals from model assumptions.
Table 6.
Models of blood manganese levels with competing Mn metrics using a half-life of 65 days for Mn duration and exposure contributions.
| Model | Estimate | t | p | |
|---|---|---|---|---|
| 1 | Intercept (R2 = 0.0253) | 0.880 | 26.30 | <0.0001 |
| Age-40 | 0.006 | 0.97 | 0.33 | |
| (Age-40)2 | −4 × 10−4 | −0.92 | 0.36 | |
| Education beyond 12 yr | −0.023 | −1.78 | 0.08 | |
| (Education beyond 12 yr)2 | −0.001 | −0.38 | 0.70 | |
| 2 | Intercept (R2 = 0.2148) | 0.723 | 19.65 | <0.0001 |
| Age-40 | −0.001 | −0.25 | 0.81 | |
| (Age-40)2 | 3.4 × 10−5 | 0.08 | 0.934 | |
| Education beyond 12 yr | −0.0170 | −1.43 | 0.15 | |
| (Education beyond 12 yr)2 | 8.2 × 10−4 | 0.33 | 0.74 | |
| Duration | 1.34 | 7.42 | <0.0001 | |
| 3 | Intercept (R2 = 0.2162) | 0.761 | 22.36 | <0.0001 |
| Age-40 | 0.001 | 0.09 | 0.93 | |
| (Age-40)2 | 9 × 10−4 | 0.19 | 0.85 | |
| Education beyond 12 yr | −0.015 | −1.28 | 0.20 | |
| (Education beyond 12 yr)2 | −0.001 | −0.54 | 0.59 | |
| Small resp. particulate (Mn-SRP) | 47.25 | 7.45 | <0.0001 | |
| 4 | Intercept (R2 = 0.2163) | 0.761 | 22.28 | <0.0001 |
| Age-40 | 0.001 | 0.11 | 0.91 | |
| (Age-40)2 | 8 × 10−5 | 0.19 | 0.85 | |
| Education beyond 12 yr | −0.015 | −1.28 | 0.20 | |
| (Education beyond 12 yr)2 | −0.001 | −0.56 | 0.58 | |
| Large resp. particulate (Mn-LRP) | −1.410 | −0.16 | 0.87 | |
| Small resp. particulate (Mn-SRP) | 47.87 | 6.45 | <0.0001 | |
| 5 | Intercept (R2 = 0.2363) | 0.727 | 19.98 | <0.0001 |
| Age-40 | −0.001 | −0.20 | 0.84 | |
| (Age-40)2 | 1 × 10−4 | 0.26 | 0.79 | |
| Education beyond 12 yr | −0.015 | −1.30 | 0.20 | |
| (Education beyond 12 yr)2 | −2 × 10−4 | −0.07 | 0.94 | |
| Duration | 0.732 | 2.45 | 0.02 | |
| Small resp. particulate (Mn-SRP) | 26.58 | 2.53 | 0.01 | |
| 6 | Intercept (R2 = 0.2334) | 0.731 | 20.75 | <0.0001 |
| Age-40 | −0.001 | −0.18 | 0.86 | |
| (Age-40)2 | 2 × 10−4 | 0.37 | 0.71 | |
| Education beyond 12 yr | −0.015 | −1.28 | 0.20 | |
| (Education beyond 12 yr)2 | −3 × 10−4 | −0.11 | 0.91 | |
| Mn-SRP (cum. sq. root) | 9.147 | 7.87 | <0.0001 | |
| 7 | Intercept (R2 = 0.2334) | 0.731 | 20.68 | <0.0001 |
| Age-40 | −0.001 | −0.19 | 0.85 | |
| (Age-40)2 | 2 × 10−4 | 0.37 | 0.71 | |
| Education beyond 12 yr | −0.015 | −1.27 | 0.20 | |
| (Education beyond 12 yr)2 | −2 × 10−4 | −0.10 | 0.92 | |
| Mn-LRP (cum. sq. root) | 0.099 | 0.06 | 0.95 | |
| Mn-SRP (cum. sq. root) | 9.102 | 6.50 | <0.0001 | |
| 8 | Intercept (R2= 0.2355) | 0.724 | 19.90 | <0.0001 |
| Age-40 | −0.001 | −0.24 | 0.81 | |
| (Age-40)2 | 2 × 10−4 | 0.34 | 0.73 | |
| Education beyond 12 yr | −0.015 | −1.30 | 0.20 | |
| (Education beyond 12 yr)2 | 4 × 10−5 | 0.02 | 0.99 | |
| Duration | 0.347 | 0.79 | 0.43 | |
| Mn-SRP (cum. sq. root) | 7.078 | 2.47 | 0.01 |
Blood Mn, B-Mn, as µg/dL; p-value: two-tailed.
Model: B-Mn = α + β(education-12)+ γ(education-12)2 + δ(age-40) + ε(age-12)2 + η(metX1) + υ(metX2), where metXi is an exposure metric: duration, Mn-SRP, etc.
Cumulative metrics for Mn-TRP, Mn-LRP and Mn-SRP in mg/m3-yr and duration in yr (with 65 day half-life applied).
With exclusions for missing data on B-Mn, education, age or work history, n: 103 Mn-alloy production workers, 131 non-exposed workers.
4. Discussion
4.1. Fume as the dominant exposure
The observation that duration of Mn exposure (with a half-life applied) was comparable to the better burden metrics in predicting B-Mn suggests that the relevant exposure is widely and somewhat uniformly dispersed spatially and over time. In this ferro- and silico-alloy facility, this finding would point to Mn condensation fume from the furnace as the most likely source. The observed superior prediction with the derived Mn-SRP metric and the much inferior prediction with the Mn-LRP metric supports this conclusion. Manganese fume is known to form aggregates of primary particles fused together and agglomerates which are clusters of primary particles that adhere via electrostatic forces (Jenkins et al., 2005). The clusters are often in the form of strings, and the spatial dispersion of the small particles within the agglomerates would result in lower sedimentation rates, more like those of the small spherical particles comprising the agglomerates.
Alternatively, the uptake and tissue distribution of Mn from the lungs may be non-linear, increasing much less than proportionally with increasing air concentrations. This would occur if homeostatic metabolic regulation was limiting blood and tissue level Mn excursions. Observing a dose-rate effect with square root of Mn-SRP supports this hypothesis, as does the reduction in the joint contribution of duration when the square root is applied in calculating Mn-SRP for predicting B-Mn (Table 6; models 5 vs. 8).
The observed optimum half-life of about 65 days for predicting Mn blood levels is consistent with reported brain and bone clearance half-life (“half-lives greater than 50 days,” as summarized in Andersen et al., 1999). However, half-life in this context is complicated, reflecting different tissue-specific clearance rates including the release of Mn irreversibly bound to red blood cells, which live 100–120 days (H. Clewell, September 24, 2013, private correspondence).
Extensive investigations of animal and human data and the development of physiologically-based pharmacokinetic (PBPK) models have revealed a complex metabolic regulation of Mn tissue levels and demonstrate dose-dependent clearance rates that would tend to moderate tissue level excursions even with highly variable inhalation exposures (Andersen et al., 2010; Schroeter et al., 2011). Whether this PBPK model would imply piece-wise linear regions in the relationship between air concentrations and brain-tissue concentrations, with a smaller slope in the region of metabolic control, cannot be discerned from Schroeter et al. (2011). At higher exposures the relationship is concave downward due to biliary induction (H. Clewell, September 24, 2013, private correspondence). Under the exposure conditions of the current study (air concentrations generally <0.2 mg/m3 small respirable Mn particulate), duration of exposure over a broad range of exposure levels (0.01–0.4 mg/m3, with a half-life applied), appears to be an efficacious exposure metric for predicting B-Mn, although not as good as the small respirable particulate (square root) metric.
Misclassification in the Mn-SRP metric would tend to diminish its relative predictive ability compared to duration, a precisely known entity. The superior prediction with calculation of burdens using square root of fume exposure intensity could represent a reduction in misclassification if the errors were greater at extreme values of the exposure metric.
Several aspects of Mn exposure as examined here may be important in explaining inconsistencies across studies in predicting B-Mn levels. Differences in the time course of exposure may be partially accommodated with application of a half-life weighting even though it represents a crude physiological approximation. A dose-rate effect, possibly related to homeostasis, and attention to the size, structure and solubility of Mn-containing respirable particulates could also bring some coherence to conflicting observations. The LRP and SRP exposures may have important differences not only on particle size, but also on process-related features such as surface oxide composition and the ratio of surface area to mass that could affect solubility and peak levels of Mn in blood under conditions of time-variable exposure. Application of metrics addressing these issues in other populations is needed for validation. This study does not imply that current B-Mn is itself an appropriate predictor of health effects.
The different associations between B-Mn and respirable Mn in dust and fumes for this Mn alloy facility has implications for other populations. It suggests that welders may receive higher biologically effective doses than workers exposed to similar concentrations of respirable dusts consisting of larger non-agglomerated particles and that the size distribution of sub-micron dusts may be important (Jenkins et al., 2005; Zimmer and Biswas, 2001). Therefore, in investigating neurobehavioral effects in Mn-exposed worker populations, exposure metrics attentive to sub-micron size distribution would be appropriate. Although representing probably very different physiological processes, the uptake of lead (Pb) into the blood has also been observed to be higher than expected when present as a furnace-generated fume compared to larger-particulate dusts generated mechanically (Froines et al., 1986) or when large particles are less prevalent (Hodgkins et al., 1992).
4.2. Limitations of study
The derivation of Mn dust and fume levels depended on a reconstruction of respirable Mn levels from historical total dust measurements to which were applied estimates of the respirable proportion and Mn composition across job groups. These estimates were based entirely on surveys performed in 1991 and assumed that the ratio of respirable to total Mn was characteristic of a given area and stable over time and that the area ratio was applicable to personal exposure for job groups located in that area (Baldwin et al., 2008). Given the available data, as in any retrospective exposure assessment, this procedure undoubtedly entailed considerable exposure misclassification. Assumptions made in partitioning the dust and fume components could have introduced further misclassification. As a consequence of the rapid decline over time of the contribution of a Mn exposure to future B-Mn, the exposures determining model fit were largely recent ones – within prior 2 years. In this study these later exposures were more dominated by fume than earlier due to the closing of the sinter operation, although in the product crushing area, dust levels remained quite uniform over time. The striking difference in predictive ability between Mn-SRP and Mn-LRP is unlikely to have resulted largely from misclassification, a condition that typically degrades prediction rather than strengthening it.
Acknowledgements
Fabrice Larribe assisted in data file retrieval and documentation. Kevin W. Hanley, Dr. Neal Zimmerman and Dr. Harvey J. Clewell III provided helpful critiques.
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
Transparency document
The Transparency document associated with this article can be found in the online version.
References
- Andersen ME, Dorman DC, Clewell HJ, III, Taylor MD, Nong A. Multi-dose-route, multi-species pharmacokinetic models for manganese and their use in risk assessment. J Toxicol Environ Health. 2010;73(Part A):217–234. doi: 10.1080/15287390903340849. [DOI] [PubMed] [Google Scholar]
- Andersen ME, Gearhart JM, Clewell HJ., III Pharmacokinetic data needs to support risk assessments for inhaled and ingested manganese. Neurotoxicology. 1999;20:161–172. [PubMed] [Google Scholar]
- Baldwin M, Bouchard M, Larribe F, Mergler D. Past occupational exposure to airborne manganese in a manganese alloy plant. J Occup Environ Hygiene. 2008;5:426–437. doi: 10.1080/15459620802115831. [DOI] [PubMed] [Google Scholar]
- Bouchard M, Mergler D, Baldwin M, Panisset M, Bowler R, Roels H. Neurobehavioral functioning after cessation of manganese exposure: a follow-up after 14 years. AJIM. 2007a;50:831–840. doi: 10.1002/ajim.20407. [DOI] [PubMed] [Google Scholar]
- Bouchard M, Mergler D, Baldwin M, Panisset M, Roels A. Neuropsychiatric symptoms and past manganese exposure in a ferro-alloy plant. Neurotoxicology. 2007b;28:290–297. doi: 10.1016/j.neuro.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Bouchard M, Mergler D, Baldwin M, Panisset M. Manganese cumulative exposure and symptoms: a follow-up study of alloy workers. Neurotoxicology. 2008;29:577–583. doi: 10.1016/j.neuro.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Elder A, Gelein R, Silva V, Feikert T, Opanashuk L, Carter J, et al. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ Health Perspect. 2006;114:1172–1178. doi: 10.1289/ehp.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingsen DG, Hetland SM, Thomassen Y. Manganese air exposure assessment and biological monitoring in the manganese alloy production industry. J Environ Monit. 2003;5:84–90. doi: 10.1039/b209095c. [DOI] [PubMed] [Google Scholar]
- Froines JR, Liu W-CV, Hinds WC, Wegman DH. Effect of aerosol size on the blood lead distribution of industrial workers. Am J Ind Med. 1986;9:227–237. doi: 10.1002/ajim.4700090305. [DOI] [PubMed] [Google Scholar]
- Guillarte TR. Manganese and parkinson’s disease: a critical review and new findings. Environ Health Perspect. 2010;118:1071–1080. doi: 10.1289/ehp.0901748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkins DG, Robins TG, Hinkamp DL, Schork MA, Krebs WH. A longitudinal study of the relation of lead in blood to lead in air concentrations among battery workers. Br J Ind Med. 1992;49:241–248. doi: 10.1136/oem.49.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins NT, Pierce WM, Eagar TW. Particle size distribution of gas metal and flux core arc welding fumes. Welding J. 2005 Oct;84(Suppl.):156s–163s. [Google Scholar]
- Lucchini R, Apostoli P, Pierrone C, Placida D, Albini E, Migliorati P, et al. Long term exposure to low levels of manganese oxides and neurofunctional changes in ferroalloy workers. Neurotoxicology. 1999;20:287–298. [PubMed] [Google Scholar]
- Lucchini R, Sellis L, Folli D, Apostoli P, Mutti A, Vanoni O, Iregren A, Alessio L. Neurobehavioral effects of manganese in workers from a ferroalloy plant after temporary cessation of exposure. Scand J Work Environ Health. 1995;21:143–149. doi: 10.5271/sjweh.1369. [DOI] [PubMed] [Google Scholar]
- Mergler D, Baldwin M. Early manifestations of manganese neurotoxicity in humans: an update. Environ Res. 1997;73:92–100. doi: 10.1006/enrs.1997.3710. [DOI] [PubMed] [Google Scholar]
- Mergler D, Baldwin M, Bélanger S, Larribe F, Beuter A, Bowler R, Panisset M, Edwards R, de Geoffroy A, Sassine MP, Hudnell K. Manganese neurotoxicity, a continuum of dysfunction: results from a community based study. Neurotoxicology. 1999;20:327–342. [PubMed] [Google Scholar]
- Mergler D, Huel GG, Bowler RR, Iregren AA, Belanger SS, Baldwin MM, et al. Nervous system dysfunction among workers with long-term exposure to manganese. Environ Res. 1994;64:151–180. doi: 10.1006/enrs.1994.1013. [DOI] [PubMed] [Google Scholar]
- Myers JE, Thompson ML, Ramushu S, Young T, Jeebhay MF, London L, et al. The nervous system effects of occupational exposure on workers in a South African manganese smelter. Neurotoxicology. 2003;24:885–894. doi: 10.1016/S0161-813X(03)00081-0. [DOI] [PubMed] [Google Scholar]
- Park RM, Bowler RM, Roels HA. Exposure-response relationship and risk assessment for cognitive deficits in early welding-induced manganism. J Occup Environ Med. 2009;51:1125–1136. doi: 10.1097/JOM.0b013e3181bd8114. [DOI] [PubMed] [Google Scholar]
- Roels H, Lauwerys R, Buchet JP, Genet P, Sarhan MJ, Hanotiau I, et al. Epidemiological survey among workers exposed to manganese: effects on lung, central nervous system, and some biological indices. Am J Ind Med. 1987;11:307–327. doi: 10.1002/ajim.4700110308. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. Cary, NC, USA: SAS Institute; 2011. [Google Scholar]
- Schroeter JD, Nong A, Yoon M, Taylor MD, Dorman DC, Andersen ME, et al. Analysis of manganese tracer kinetics and target tissue dosimetry in monkeys and humans with multi-route physiologically based pharmacokinetic models. Toxicol Sci. 2011;120:481–498. doi: 10.1093/toxsci/kfq389. [DOI] [PubMed] [Google Scholar]
- Smith D, Gwiazda R, Bowler R, Roels H, Park R, Taicher C, et al. Biomarkers of Mn exposure in humans. Am J Ind Med. 2007;50:801–811. doi: 10.1002/ajim.20506. [DOI] [PubMed] [Google Scholar]
- Sunderman FW., Jr Nasal toxicity, carcinogenicity, and olfactory uptake of metals. Ann Clin Lab Sci. 2001;31:3–24. [PubMed] [Google Scholar]
- Zheng W, Fu SX, Dydak U, Cowan DM. Biomarkers of manganese intoxication. Neurotoxicology. 2011;32:1–8. doi: 10.1016/j.neuro.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer AT, Biswas P. Characterization of the aerosols resulting from arc welding processes. J Aerosol Sci. 2001;32:993–1008. [Google Scholar]
- Zoni S, Albibi E, Lucchini R. Neuropsychological testing for the assessment of manganese neurotoxicity: a review and a proposal. Am J Ind Med. 2007;50:812–830. doi: 10.1002/ajim.20518. [DOI] [PubMed] [Google Scholar]